Is the existence of life on Earth a lucky fluke or an inevitable consequence of the laws of nature? Is it simple for life to emerge on a newly formed planet, or is it the virtually impossible product of a long series of unlikely events? Advances in fields as disparate as astronomy, planetary science and chemistry now hold promise that answers to such profound questions may be around the corner. If life turns out to have emerged multiple times in our galaxy, as scientists are hoping to discover, the path to it cannot be so hard. Moreover, if the route from chemistry to biology proves simple to traverse, the universe could be teeming with life.
The discovery of thousands of exoplanets has sparked a renaissance in origin-of-life studies. In a stunning surprise, almost all the newly discovered solar systems look very different from our own. Does that mean something about our own, very odd, system favors the emergence of life? Detecting signs of life on a planet orbiting a distant star is not going to be easy, but the technology for teasing out subtle “biosignatures” is developing so rapidly that with luck we may see distant life within one or two decades.
To understand how life might begin, we first have to figure out how—and with what ingredients—planets form. A new generation of radio telescopes, notably the Atacama Large Millimeter/submillimeter Array in Chile’s Atacama Desert, has provided beautiful images of protoplanetary disks and maps of their chemical composition. This information is inspiring better models of how planets assemble from the dust and gases of a disk. Within our own solar system, the Rosetta mission has visited a comet, and OSIRIS-REx is on its way back to Earth with samples from an asteroid, which might give us the essential inventory of the materials that came together in our planet.
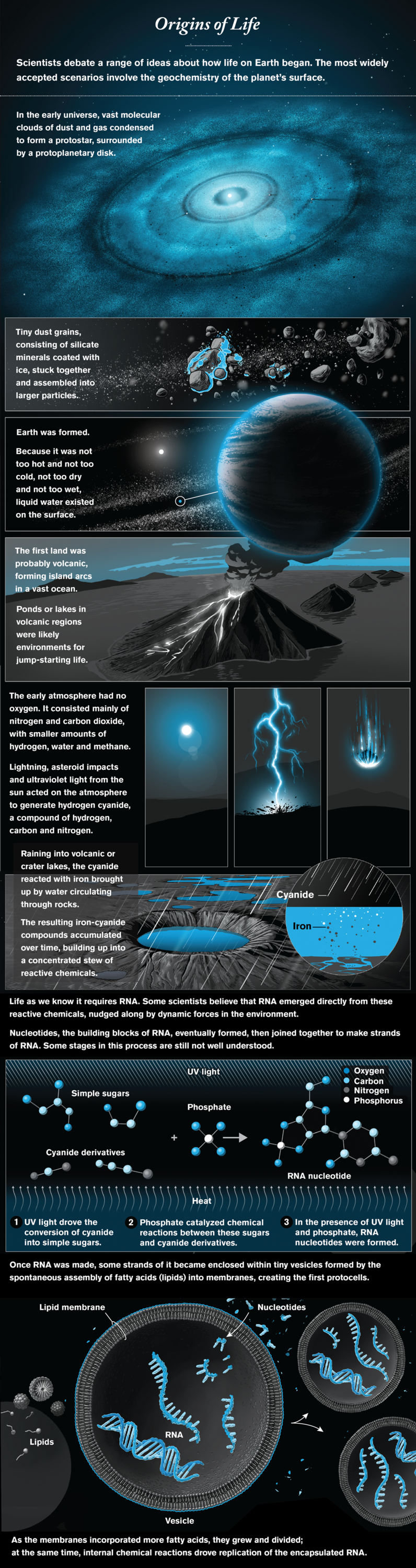
Once a planet like our Earth—not too hot and not too cold, not too dry and not too wet—has formed, what chemistry must develop to yield the building blocks of life? In the 1950s the iconic Miller-Urey experiment, which zapped a mixture of water and simple chemicals with electric pulses (to simulate the impact of lightning), demonstrated that amino acids, the building blocks of proteins, are easy to make. Other molecules of life turned out to be harder to synthesize, however, and it is now apparent that we need to completely reimagine the path from chemistry to life. The central reason hinges on the versatility of RNA, a very long molecule that plays a multitude of essential roles in all existing forms of life. RNA can not only act like an enzyme but also store and transmit information. Remarkably, all the protein in all organisms is made by the catalytic activity of the RNA component of the ribosome, the cellular machine that reads genetic information and makes protein molecules. This observation suggests that RNA dominated an early stage in the evolution of life.
Today the question of how chemistry on the infant Earth gave rise to RNA and to RNA-based cells is the central question of origin-of-life research. Some scientists think that life originally used simpler molecules and only later evolved RNA. Other researchers, however, are tackling the origin of RNA head-on, and exciting new ideas are revolutionizing this once quiet backwater of chemical research. Favored geochemical scenarios involve volcanic regions or impact craters, with complex organic chemistry, multiple sources of energy, and dynamic light-dark, hot-cold and wet-dry cycles. Strikingly, many of the chemical intermediates on the way to RNA crystallize out of reaction mixtures, self-purifying and potentially accumulating on the early Earth as organic minerals—reservoirs of material waiting to come to life when conditions change.
Assuming that key problem is solved, we will still need to understand how RNA was replicated within the first primitive cells. Researchers are just beginning to identify the sources of chemical energy that could enable the RNA to copy itself, but much remains to be done. If these hurdles can also be overcome, we may be able to build replicating, evolving RNA-based cells in the laboratory—recapitulating a possible route to the origin of life.
What next? Chemists are already asking whether our kind of life can be generated only through a single plausible pathway or whether multiple routes might lead from simple chemistry to RNA-based life and on to modern biology. Others are exploring variations on the chemistry of life, seeking clues as to the possible diversity of life “out there” in the universe. If all goes well, we will eventually learn how robust the transition from chemistry to biology is and therefore whether the universe is full of life-forms or—but for us—sterile.
This article is part of a special report, “The Biggest Questions in Science,” sponsored by The Kavli Prize. It was produced independently by Scientific American and Nature editors, who have sole responsibility for all the editorial content.



