In the past few years millions of people around the world have radically changed their way of life to avoid contact with other people and, thus, the novel coronavirus. Despite social distancing, many have still gotten sick in part from other viral infections. That is because, as scientists are increasingly learning, many viruses are lurking quietly in the human body, hidden away in cells in the lungs, blood and nerves and inside the multitudes of microbes that colonize our guts.
Biologists estimate that 380 trillion viruses are living on and inside your body right now—10 times the number of bacteria. Some can cause illness, but many simply coexist with you. In late 2019, for example, researchers at the University of Pennsylvania discovered 19 different strains of redondovirus in the respiratory tract; a handful of them were associated with periodontal disease or lung disease, but others could possibly fight respiratory illnesses. Scientists’ rapidly expanding knowledge makes it clear that we are not made up primarily of “human” cells that are occasionally invaded by microbes; our bodies are really superorganisms of cohabitating cells, bacteria, fungi and, most numerous of all, viruses. The latest counts indicate that as much as half of all the biological matter in your body is not human.
A decade ago researchers were barely aware that the human virome existed. Today we see the vast virome as an integral part of the larger human microbiome, a crazy quilt of passive and active microscopic organisms that occupy almost every corner of our being. We have been mapping the virome for more than 10 years, and the deeper we investigate, the more the virome looks like a partnership that can influence our daily lives positively as well as negatively. Recent research shows we could even harness the virome to promote our own health. Investigators at the Rockefeller University, for example, purified an enzyme from a virus that kills bacteria found in patients who are struggling against methicillin-resistant staphylococcal infection. The results were so encouraging that the Food and Drug Administration designated the enzyme as a “breakthrough therapy.” Today we routinely speak about the “good” and “bad” bacteria in our lives. Viruses fall into the same categories. The challenge now is to figure out how to stop the bad ones and promote the good ones.
Infected at Birth
The human body is a rich environment for microbes, replete with proteins, fats and carbohydrates. Many viruses have figured out how to peacefully thrive in it without making us sick.
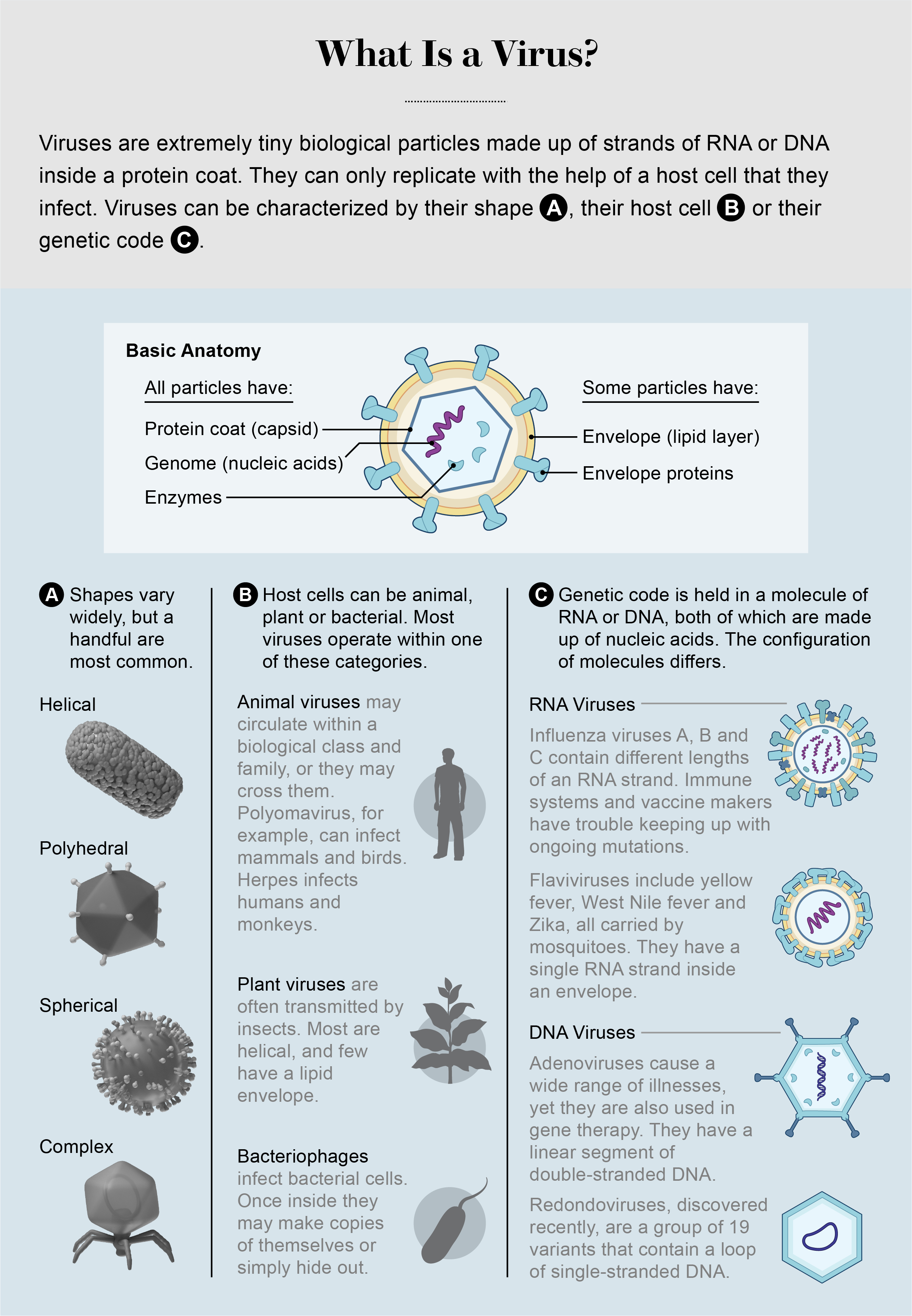
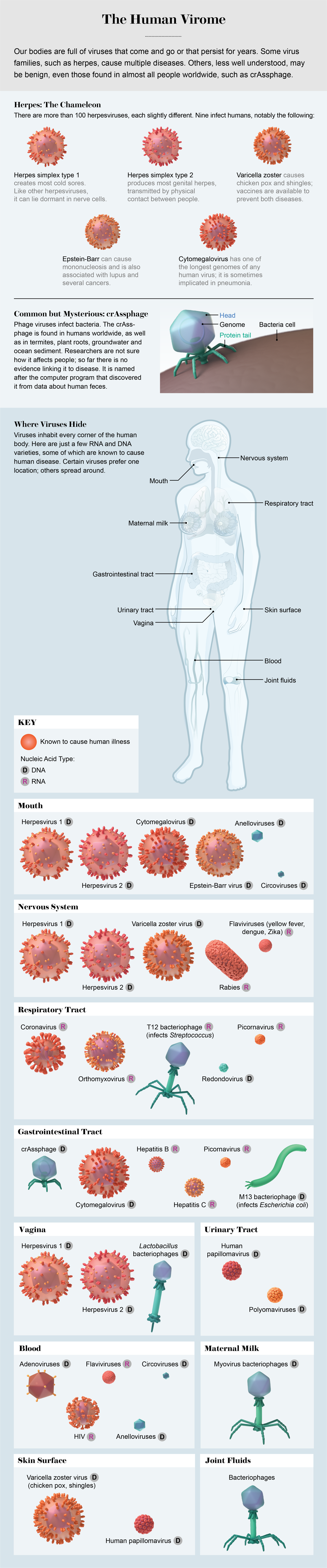
Viruses need to invade host cells to reproduce, and they are adept at exploiting all the options in our bodies. About a dozen years ago inexpensive genome sequencing led us to discover plentiful viruses in the mouth and gut. By 2013 or so scientists located viruses on the skin and in the respiratory tract, blood and urine. Most recently, we have found them in even more surprising places. In September 2019, for example, Chandrabali Ghose and our colleagues and I published details about viruses that we discovered in the cerebrospinal fluid of adults who were undergoing testing for various conditions. The viruses belonged to several different families and were not associated with any known disease. We also found the same viruses in blood plasma, joint fluid and breast milk. Scientists knew that a few rare, infectious viruses, notably herpes, could sneak into cerebrospinal fluid, but finding random viruses that seemed to be mere bystanders was a surprise. The central nervous system, which is supposed to be a sterile environment, is colonized by a somewhat diverse viral community.
It appears that our viromes begin to accumulate when we are born. Studies reveal a high diversity of viruses in the infant gut shortly after birth, suggesting that they probably come from the babies’ mothers, some ingested from breast milk. Some of these viruses decline in number as infants grow to weeks or months old; others enter their bodies from the air, water, food and other people. These viruses grow in number and diversity, infecting cells where they will persist for years. Infant viromes are unstable, whereas adult viromes are relatively stable. Anelloviruses, a family of 200 different species, are present in almost everyone as we get older. This mirrors what we observe for bacteria as well.
Many of the viruses living inside us do not target our cells. Instead they look for the bacteria in our microbiomes. Known as bacteriophages, or phages, these viruses sneak inside bacterial cells, use the machinery there to make copies of themselves, then often burst out to infect more bacteria, killing their host cells in the process. Bacteriophages are nearly ubiquitous in nature. If you look hard enough, you will find them in soil, in any source of water from the ocean to your tap at home, and in extreme environments such as acid mines, the Arctic and hot springs. You will even find them floating in the air. They persist in all these places because they are hunting the bacteria that live in all these places. We humans are just another hunting ground.
In 2017 Sophie Nguyen and Jeremy Barr, then at San Diego State University, demonstrated that many phages get to their final locations in the body by crossing through mucosal membranes. In laboratory experiments, phages worked through membranes that line the intestine, lung, liver, kidney and even the brain. But when they randomly cross into a place such as the central nervous system, where there are few bacteria to be hosts, they may have no way to replicate and may ultimately perish.
Your Personal Virus Profile
The virome can vary greatly from one part of the body to another. When Ghose and I looked for viruses in unexpected places, we also determined that viruses in the mouth are different from viruses in the gut, which are different from viruses in urine or in blood. We knew this was the case for bacteria, but early on we did not have enough data for viruses. Although it is not difficult to find volunteers who will spit in a cup, it is hard to get them to provide stool or blood samples and to persuade universities to sign off on obtaining and processing these samples. When we do have the goods, we must filter out the bacteria, leaving tiny bits of viral material we can examine under a microscope and insert into a machine that sequences the nucleic acids that encode the genes that are present. Still, researchers have done enough of this work now to be able to tell what part of the body they are examining just by noting the viruses present.
My colleague Melissa Ly of the Duke Human Vaccine Institute and I have also shown that by comparing the viromes of unrelated people, we can determine if any of them live together. Although different people can have significantly different viromes, people who cohabitate appear to share about 25 percent of the viruses in their viromes. Viruses can be transmitted from one household member to another not just through typical contagious means such as coughing but also through casual contact and sharing sinks, toilets, desks and food. Although we have studied only small numbers of people, the data show that nonromantic roommates share a similar percentage of viruses as romantic roommates do. Intimate contact seems to make little difference; just living in the same space is enough.
The puzzle is tricky, however. Shira Abeles of the University of California, San Diego, has identified big differences in the oral viromes of men and women; hormones could be the reason, but no one has demonstrated such a connection. We do know that viromes can vary considerably with geographic populations. For example, there is less diversity in the viromes of individuals in Western countries than there is among individuals in non-Western countries. These differences may be related to both diet and environment.
Vagabonds or Freeloaders?
Many viruses in our virome infect bacteria, but a smaller proportion infect cells in our tissues directly. These viruses may be in the minority because our immune system suppresses them. Iwijn De Vlaminck, then at Stanford University, demonstrated that when a person’s immune system is strongly challenged—for example, when someone has received an organ transplant and must take immunosuppressing drugs to avoid rejecting the organ—the presence of certain viruses increases dramatically. In these cases, we see a rise in both viruses known to cause disease and those that do not. This observation suggests that under normal circumstances our immune system keeps the virome in check, but when immunity is hampered, viruses can multiply readily.
We may be seeing this kind of opportunism with COVID-19. People who get sick from the SARS-CoV-2 virus, particularly those with severe illness, may develop coinfections. The most common are a secondary bacterial pneumonia, or bacteremia (a rise of bacteria in the bloodstream), involving organisms such as Staphylococcus aureus and Streptococcus pneumoniae. Though less common, we have also seen viral coinfections such as influenza, respiratory syncytial virus and adenovirus. Viruses lurking in the virome may also reactivate, such as Epstein-Barr virus and cytomegalovirus. When the immune system is paying attention to COVID, the patient may be more susceptible to other viral outbreaks.
Many phages, despite being hunters, live in harmony with their prey for a long time and may never break out. A virus is just a ball of protein enveloping a molecule of genetic instructions—the virus’s genetic code. When some phages infect a bacterium, they integrate their genome into the bacterium’s genome. Although certain viruses reproduce immediately, killing their host bacteria, other phages just persist inside their host, as if in quiet hibernation. This is probably a survival strategy; when the host bacterium divides, creating a copy of its genome, it copies the phage genome as well. In this model, the survival of the host determines the survival of the phage, so the phage has a vested interest in maintaining its host. It is clear why such a strategy benefits the phage but not so clear how it could benefit the bacteria. For whatever reason, it seems that many bacteria in the body have grown accustomed to living with their phages.
When the opportunity arises, hibernating phages may awaken and produce many progeny, killing their host cells. Sometimes the exiting phages take bacterial genes along with them. This payload can at times benefit the next bacteria the phages infect. I have found phages in saliva, for example, carrying genes that help bacteria evade our immune system. Some phages even carry genes that help bacteria resist antibiotics. Phages have no need for such genes, because phages cannot be killed by antibiotics, so when they provide the genes to bacteria they promote the hosts’ survival—synonymous with survival of the phages. We see these kinds of transfers often.
Phages can take protection of their host further. The bacterium Pseudomonas aeruginosa, best known for causing pneumonia, triggers a number of illnesses. People who have lung diseases such as cystic fibrosis find it nearly impossible to clear this bacterium from their lungs, even when taking antibiotics designed to kill it. Some P. aeruginosa have integrated what are called filamentous phages into their genomes. In 2019 researchers led by a group at Stanford, including Elizabeth Burgener and Paul Bollyky, discovered that filamentous phages can form a protective cloak—layers of carbohydrates and proteins that help bacteria hide from antibiotics. This allows the bacteria to shelter in place until the antibiotics go away, living to cause infection another day.
Viruses That Help Us
It is not a big leap to wonder whether we can harness the viruses living within us to improve our health. We have already found a few cases in which this happens naturally. As phages move around the body looking for bacteria, some of them bind to cells on the surface of mucosal membranes, such as those that line the nose, throat, stomach and intestines. The phages cannot replicate there, but they can lie in wait for a vulnerable host to come by.
This process could theoretically protect us from some illnesses. Say you eat food contaminated with Salmonella bacteria. If the bacteria brush along the stomach’s membrane, phages there could ostensibly infect the bacteria and kill them before they can cause disease. In this way, phages may serve as a de facto immune system that protects us against disease. No one has proved this yet, but in 2019 a research group in Finland showed that phages bound to mucus in pigs and rainbow trout persisted there for seven days and protected against one kind of bacterium that infects these animals.
One phage getting a lot of attention is crAssphage, discovered in 2014 by Bas Dutilh of the Radboud Institute in the Netherlands. Studies since then have shown that it inhabits most people around the world—except, it seems, for traditional hunter-gatherer populations. It is unusual to find the exact same virus spread so far and wide, and no one has linked it to any disease. Scientists think it controls the prevalence of a common gut bacterium called Bacteroides. If so, we might be able to exploit it to improve gastrointestinal conditions. It is so prevalent in human feces that researchers now test for it in drinking water to see if the water has been contaminated by sewage.
Physicians are especially interested in phages that might counteract the aggressive rise of antibiotic-resistant bacteria. Development of new antibiotics has failed to keep pace. The World Health Organization has estimated that by 2050 these pathogens will cause at least 10 million deaths annually, so alternative therapies are vital. Phages were discovered more than 100 years ago, and physicians tried to use them to treat bacteria that cause disease, though without great success. In the 1940s antibiotics replaced phages in most of the world because the drugs were much more effective and much easier to use. Now some medical researchers, such as the Rockefeller University investigators who used a phage enzyme to fight methicillin-resistant Staphylococcus infection, are taking a new look at phages.
For years most physicians have been afraid to administer phages because they did not know whether the human immune system would overreact, causing dangerous levels of inflammation. Phages for therapeutic use are grown in bacteria, and if the bacteria are not completely removed before the phages are administered, the bacteria can trigger an overly aggressive immune response. Today we have more sophisticated methods of purifying phages, and worries about adverse reactions have largely subsided.
What really limits the use of phages to treat infectious disease is that effective viruses are hard to find. For many years researchers have combed through natural habitats for phages that might be active against bacteria that cause human disease. Now that we know viruses are plentiful in feces, saliva and sputum, researchers have realized that one of the richest sources of phages may be local sewage-processing plants.
A few such phages are already being used for experimental treatments. In a landmark 2016 case overseen by Robert Schooley, also at U.C. San Diego, doctors used phages from sewage, as well as those from environmental sources, to successfully treat Tom Patterson, a professor at the school who was in multiorgan failure because of Acinetobacter baumannii, a notoriously drug-resistant bacteria.
Improved Health
As we learn more about the roles of viruses in the human virome, we may uncover more therapeutic possibilities. Alejandro Reyes of Washington University in St. Louis has shown that phages in mice can shape the rodents’ bacterial communities, although we are not sure what changes first: the viruses or the bacteria. If the viral communities change first, they can sculpt the bacterial communities to serve them. If the bacterial communities change first, the viral communities are probably just adapting so they can infiltrate the reshaped bacteria. Researchers have shown that viromes can change significantly in periodontal disease and in inflammatory bowel diseases.
Although it will take a long time for us to unravel the human virome, it is important to consider how much progress we made in just 10 years. A decade ago many scientists thought of the microbiome as a kind of passive layer of tiny organisms inside the body, mostly in the gut. Now we know that although some parts of the microbiome are indeed stable, some parts are active and changing. And it is beginning to look like the most dynamic players are the viruses. A 2018 study of brain tissue donated by people who had died of Alzheimer’s disease revealed high levels of herpesviruses. Then, in May 2020, investigators at Tufts University and the Massachusetts Institute of Technology, who have developed brainlike tissue in the lab, infected their tissue with herpes simplex 1, and the tissue became full of amyloid plaquelike formations akin to those that riddle the brains of people who have Alzheimer’s. It is startling to realize that we could discover remarkable roles for old viruses.
As we look deeper, we may find new categories of viruses that impact human health, as well as new ways to exploit viruses to manipulate our microbiome and protect us from disease. If we humans can figure out how to manage the bad viruses and exploit the good ones, we could help ourselves become stronger superorganisms.