In Aldous Huxley’s Brave New World, a boy memorizes each word of a lecture in English, a language he does not speak. The learning happens as the boy sleeps within earshot of a radio broadcast of the lecture. On awakening, he is able to recite the entire lecture. Based on this discovery, the totalitarian authorities of Huxley’s dystopian world adapt the method to shape the unconscious minds of all their citizens.
Sleep learning turns up throughout literature, pop culture and ancient lore. Take Dexter, the lead character in the animated television series Dexter’s Laboratory. In one episode, Dexter squanders his time for homework, so instead he invents a contraption for learning to speak French overnight. He wakes up the next day unable to speak anything but French. The idea of sleep learning isn’t just a modern invention. It also appears within a centuries-old mind-training practice of Tibetan Buddhists; a message whispered during sleep was intended to help a monk recognize the events in his dreams as illusory.
Everyone knows we learn better when we are well rested. Most people, however, dismiss the notion of sleep learning out of hand. Yet a set of new neuroscientific findings complicates this picture by showing that a critical part of learning occurs during sleep: recently formed memories resurface during the night, and this playback can help reinforce them, allowing at least a few to be remembered for a lifetime.
Some studies have even explored whether sleep might be manipulated to enhance learning. They reveal that sleep’s program for making daytime memories stronger can be boosted using sounds and odors. Results in rodents have even demonstrated a primitive form of memory implantation: using electrical stimulation while animals slept, researchers taught them where they should go in their enclosures on awakening. Huxley’s imagined version of sleep education, in which entire texts are absorbed verbatim during the night, is still relegated to the pages of his 1932 classic. But experiments now indicate that it is possible to tinker with memories while a person is immersed in the depths of slumber, creating the basis for a new science of sleep learning.
The PsychoPhone
For these techniques to work, scientists have to explore how information can be absorbed when consciousness is seemingly on a well-deserved break. Around the time that Huxley was writing Brave New World, serious explorations into the possibility of meddling with sleep had begun. In 1927 New Yorker Alois B. Saliger invented an “Automatic Time-Controlled Suggestion Machine,” which he marketed as the “PsychoPhone,” to allow a recorded message to be replayed during the night. The setup seemed to evoke Huxley’s imagined technology except that the user, rather than the state, could select the message to be played.
Saliger’s invention was followed, in the 1930s and 1940s, by studies documenting ostensible examples of sleep learning. A 1942 paper by Lawrence LeShan, then at the College of William & Mary, detailed an experiment in which the researcher visited a summer camp where many of the boys had the habit of biting their fingernails. In a room where 20 such boys slept, LeShan used a portable phonograph to play a voice repeating the sentence “My fingernails taste terribly bitter.” The string of words recurred 300 times each night, beginning 150 minutes after the onset of sleep. The experiment continued for 54 consecutive nights. During the last two weeks of camp, the phonograph broke, so the intrepid LeShan delivered the sentence himself. Eight of the 20 boys stopped biting their nails, whereas none of 20 others who slept without exposure to the recording did so. These early efforts did not use physiological monitoring to verify that the boys were really asleep, though, so the results remain suspect.
The whole field took a severe hit in 1956, when two scientists at RAND Corporation used electroencephalography (EEG) to record brain activity while 96 questions and answers were read to sleeping study participants. (One example: “In what kind of store did Ulysses S. Grant work before the war?” Answer: “A hardware store.”) The next day correct answers were recalled only for information presented when sleepers showed signs of awakening. These results led to a shift in the field that persisted for 50 years, as researchers began to lose faith in sleep learning as a viable phenomenon: participants in these experiments appeared to learn only if they were not really sleeping when information was presented to them.
Most scientists during this time tended to avoid the topic of sleep learning, although a few researchers did plug away at asking whether sleep assists in remembering new information. One typical study protocol probed whether overnight sleep deprivation affected recall the day after learning something new. Another asked whether remembering was better after a nap than after an equal period of time spent awake.
Various confounding factors can interfere with such studies. For example, the stress of sleep deprivation can harm cognitive functions, which then decreases memory recall. Eventually cognitive neuroscientists began to tackle these challenges by bringing together evidence from multiple research methods. A substantive foundation of evidence gradually accrued to confirm that sleep is a means of reviving memories acquired during the day, reopening the relation between sleep and memory as a legitimate area of scientific study.
Many researchers who took up the challenge focused on rapid eye movement (REM) sleep, the period when dreams are the most frequent and vivid. The guiding assumption held that the brain’s nighttime processing of memories would be tied to dreaming, but clear-cut data did not materialize. In 1983 two noted scientists—Graeme Mitchison and Francis Crick, neither a psychologist—went so far as to speculate that REM sleep was for forgetting. In a similar vein, Giulio Tononi and Chiara Cirelli, both at the University of Wisconsin–Madison, proposed that sleep could be the time for weakening connections among brain cells, making it easier for new information to be acquired the following day.
Instead of REM, some investigators focused their attention on slow-wave sleep (SWS), a period of deep slumber without rapid eye movements. In 2007 Björn Rasch, then at the University of Lübeck in Germany, and his colleagues prepared people for a sleep experiment by requiring them to learn the locations of a set of objects while simultaneously smelling the odor of a rose. Later, in their beds in the laboratory, sleeping study participants again encountered the same odor as electrical recordings confirmed one sleep stage or another. The odor activated the hippocampus, a brain area critical for learning to navigate one’s surroundings and for storing the new knowledge gained. On awakening, participants recalled locations more accurately—but only following cueing from odors that emanated during the course of slow-wave (not REM) sleep.
Targeted Memory Reactivation
In 2009 our lab extended this methodology by using sounds instead of odors. We found that sounds played during SWS could improve recall for individual objects of our choosing (instead of the recall of an entire collection of objects, as was the case in the odor study). In our procedure—termed targeted memory reactivation, or TMR—we first taught people the locations of 50 objects. They might learn to place a cat at one designated spot on a computer screen and a teakettle at another. At the same time, they would hear a corresponding sound (a meow for the cat, a whistle for the kettle, and so on).
After this learning phase, participants took a nap in a comfortable place in our lab. We monitored EEG recordings from electrodes placed on the head to verify that each individual was soundly asleep. These recordings provided intriguing data on the synchronized activity of networks of neurons in the brain’s outer layer, the cerebral cortex, that are relevant for memory reactivation [see graphic above]. When we detected slow-wave sleep, we played the meow, whistle and other sounds associated with a subset of the objects from the learning phase. Sounds were presented softly, not much louder than background noise, so the sleeper did not awaken.
On awakening, people remembered locations cued during sleep better than locations that had not been cued during sleep. Whether sounds or odors served as cues in these experiments, they apparently triggered the reactivation of spatial memories and so reduced forgetting.
At first, the auditory procedures we used were highly controversial. The received wisdom among sleep researchers held that sensory circuits in the cortex are largely switched off during sleep, except for the sense of smell. We were not swayed by this orthodox view. Instead we followed our hunch that the repeated playing of soft sounds might influence the sleeping brain and produce changes in recently stored memories.
Indeed, the same memory benefits were also found in many subsequent studies. A technique called functional magnetic resonance imaging highlighted which brain areas take part in TMR, and EEG results brought out the importance of specific brain oscillations. Two papers published in 2018—one by Scott Cairney of the University of York in England and his colleagues, the other by James Antony of Princeton University and his colleagues—linked an oscillation, the sleep spindle, with the memory benefits of TMR.
Besides boosting spatial memory, these procedures have also helped improve recall in other settings. TMR can assist in mastery of playing a keyboard melody and learning new vocabulary or grammatical rules. The technique can also help with simpler types of learning, such as adjustments in one’s body image. In conditioning experiments, TMR alters prior learning of an automatic reaction to a stimulus caused by an earlier pairing of that stimulus with an electric shock. Ongoing studies are examining still other types of recall, such as associating names with faces when first meeting new people.
As the technology evolves, TMR should be tested to see whether it could help to treat various disorders, reduce addictions or speed recovery from illness. Our lab, together with Northwestern University neurologist Marc Slutzky, is currently testing a novel rehabilitation procedure for recovering arm-movement abilities after stroke. Cue sounds are incorporated as part of the therapy and are replayed during sleep to try to accelerate relearning of lost movements. The prospects appear promising because TMR can alter similar forms of motor learning in healthy individuals.
What about Learning French?
The demonstrated ability to reinforce memories raises the question of whether new information can be loaded into a person’s brain after falling asleep, a technique that calls forth the ethical specter of mind control invoked by Brave New World. Is it going too far, though, to imagine that memories can be created surreptitiously?
Although the orthodox response to such conjectures has for many years been an unqualified no, studies by Anat Arzi, then at the Weizmann Institute of Science in Rehovot, Israel, and her colleagues demonstrated the creation of relatively simple memories using odors. In one experiment, the researchers succeeded in diminishing the desire for tobacco in smokers who were keen to quit. When asleep, study participants were exposed to two odors, cigarette smoke and rotten fish. During the next week, those who had smelled the mix of both odors lit up 30 percent less, having apparently been conditioned to associate smoking with the aversive fish odor.
Acquiring a more complex memory is not as easy, but evidence from the past decade holds tantalizing promise. Karim Benchenane of the French National Center for Scientific Research (CNRS) and his colleagues have shown how to literally change the mind—of a mouse. When they began their work, Benchenane and his team knew that when a mouse explores a new environment, neurons called place cells fire as the animal traverses specific parts of an enclosure. These same neurons discharge again during sleep as the memory is apparently replayed.
The researchers stimulated the reward system of the mouse brain (the medial forebrain bundle) precisely when place cells became spontaneously active while the animal was asleep. Amazingly, mice subsequently spent more time at the locations that corresponded to the stimulated place cells, heading there directly after they woke up. More experiments still need to disentangle whether fully formed false memories were implanted in the mice during sleep or whether they were automatically guided to those spots by a process of conditioning, without more knowledge about why they were drawn to those locations.
In 2019 Swiss researchers reported that sleepers could acquire new verbal knowledge, but this was evident only through subtle nonconscious means. More recently, we showed episodic learning during sleep with full recollection of the learning. In a multi-institutional collaboration by researchers in France, Germany, the Netherlands and the U.S., we used a variant of the TMR method to encourage lucid dreaming—a state in which people realize they are dreaming while remaining in the midst of the dream. We then showed that people could understand softly spoken questions from within these dreams and produce correct answers by signaling with their eyes, their respiration or subtle twitches in their facial muscles. Sometimes people in these experiments woke up able to recollect parts of their dreamtime Q and A. These rare occurrences convincingly document full-blown learning experienced entirely during sleep.
The boundaries of sleep hacking may continue to expand, but this research has established that a normal component of learning continues nocturnally off-line. Sleep is needed not just to help people stay alert and rejuvenated but also to reinforce memories initially acquired while they were awake. We still need to learn much more about off-line memory processing. Further work must ascertain how sleep helps learning and which brain mechanisms are engaged to preserve the most valuable memories. It is also essential to find out more about the perils of poor or inadequate sleep that might be affected by various forms of life stress, certain diseases or the experience of growing older.
A study led by Carmen Westerberg, then at Northwestern, points in the desired direction. Westerberg tested patients with the memory dysfunction that often precedes Alzheimer’s disease—amnestic mild cognitive impairment. The results documented a link between poor sleep and reduced ability to remember information after an intervening overnight delay.
All of this knowledge might help in creating programs of sleep learning to preserve memories, to speed the acquisition of new knowledge, or even to change bad habits such as smoking. Looking still further ahead, scientists might also explore whether we can gain control over our dreams, which could lead to the prospect of nightmare therapies, sleep-based problem-solving and perhaps even recreational dream travel. In a culture that already offers wrist-based sleep trackers and mail-order gene tests, we can begin to contemplate new ways to convert daily downtime into a productive endeavor—for some, a chilling prospect, and for others, another welcome opportunity for self-improvement.
The Maestros of Slumber
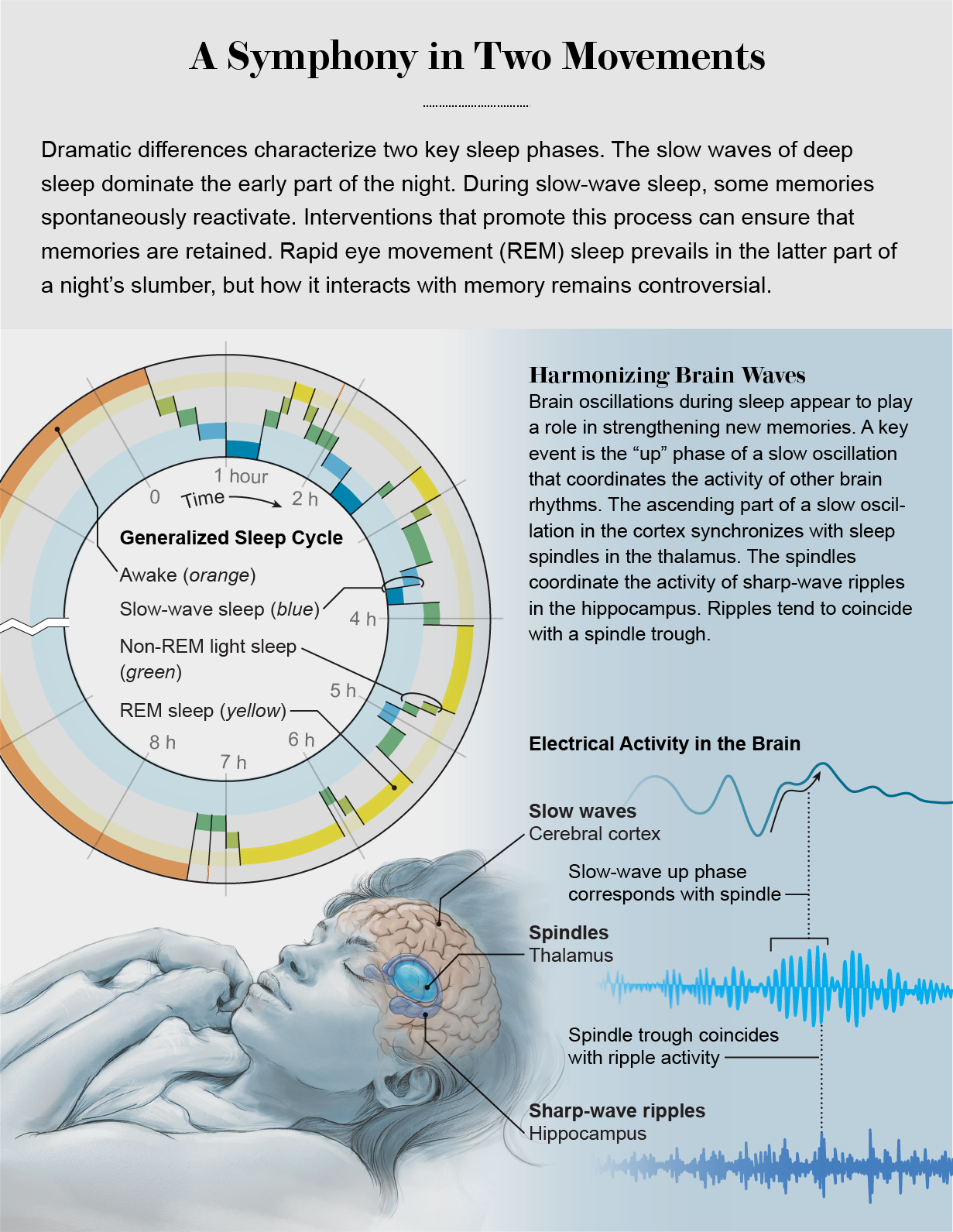
A complex symphony of neural activity governs the connection between sleep and memory
Brain rhythms provide clues to how sleep helps to store memories for later retrieval. One type of neural signal, called a slow wave, cycling from 0.5 to four times a second, orchestrates the activity of neurons in the cerebral cortex. Each slow oscillation consists of a “down” phase, when neurons are silent, and an “up” phase, when they resume activity. This timing pattern helps to reinforce recently formed memories by ensuring that multiple cortical regions remain in an up state at the same time.
The up phase can coincide with sleep spindles, brief increases of a rhythm of 12 to 15 cycles per second. Spindles originate in the thalamus, which serves as a crossroads for information that is transmitted to virtually all parts of the cerebral cortex. Spindles have a rhythm of their own, recurring at approximately five-second intervals. They coordinate the activity of sharp-wave ripples in the hippocampus. Ripples, for their part, are concurrent with the replay of memories. Slow waves, all the while, assume the role of orchestra conductor: their measured oscillations in the cortex coordinate the pacing for sleep spindles and sharp-wave ripples.
The intricate coupling of these oscillations underlies not only memory reactivation but also the altering of connections among neurons to strengthen memory storage. A dialogue between the hippocampus and the cortex involving all these brain rhythms triggers a set of complex network interactions. Through this process, known as consolidation, new information can become integrated with existing memories. The intertwining of memories, moreover, enables the gist of recent experiences to be extracted to make sense of a complex world.
Memory difficulties can arise when this neural dialogue becomes impaired. Individuals with major damage centered in the hippocampus or parts of the thalamus may develop a profound amnesia. Without the expected interactions with these brain regions during both sleep and waking, the cortex cannot store mental records of facts and events known as declarative memories. In addition, a milder form of memory disorder may result when memory processing during sleep is seriously disrupted.
Deciphering the physiological orchestration of the sleeping brain is prompting various new strategies for enhancing the brain’s natural rhythms—including stimulation with slow electrical oscillations, sounds or gentle motion. These methods echo humans’ natural inclinations to take advantage of a lullaby’s rhythm or the rocking of a cradle to lull a baby to sleep. —K.A.P. and D.O.




