Humans are willful creatures. No other species on the planet has gained so much mastery over its own fate. We have neutralized countless threats that once killed us in the millions: we have learned to protect ourselves from the elements and predators in the wild; we have developed cures and treatments for many deadly diseases; we have transformed the small gardens of our agrarian ancestors into the vast fields of industrial agriculture; and we have dramatically increased our chances of bearing healthy children despite all the usual difficulties.
Many people argue that our technological advancement—our ability to defy and control nature—has made humans exempt from natural selection and that human evolution has effectively ceased. There is no “survival of the fittest,” the argument goes, if just about everyone survives into old age. This notion is more than just a stray thought in the public consciousness. Professional scientists such as Steve Jones of University College London and respected science communicators such as David Attenborough have also declared that human evolution is over.
But it is not. We have evolved in our recent past, and we will continue to do so as long as we are around. If we take the more than seven million years since humans split from our last common ancestor with chimpanzees and convert it to a 24-hour day, the past 30,000 years would take about a mere six minutes. Yet much has unfolded during this last chapter of our evolution: vast migrations into new environments, dramatic changes in diet and a more than 1,000-fold increase in global population. All those new people added many unique mutations to the total population. The result was a pulse of rapid natural selection. Human evolution is not stopping. If anything, it is accelerating.
An Anthropological Legacy
Skeletons of ancient people have long suggested that humans evolved certain traits swiftly and recently. About 11,000 years ago, as people started to transition from hunting and gathering to farming and cooking, human anatomy changed. Ten thousand years ago, for example, people's teeth averaged more than 10 percent larger in Europe, Asia and North Africa than today. When our ancestors started to eat softer cooked foods that required less chewing, their teeth and jaws shrank, bit by bit, each generation.
Although anthropologists have known about such traits for decades, only in the past 15 years or so has it become clear just how new they really are. Studies of human genomes have made the recent targets of selection highly visible to us. It turns out, for example, that descendants of farmers are much more likely to have a greater production of salivary amylase, a key enzyme that breaks down starches in food. Most people alive today have several copies of the gene that codes for amylase, AMY1. Modern hunter-gatherers—such as the Datooga in Tanzania—tend to have far fewer copies than people whose ancestors came from farming populations, whether they live in Africa, Asia or the Americas. Getting a jump on starch processing at the point of entry seems to have been an advantage for ancient farmers wherever they adopted starchy grains.
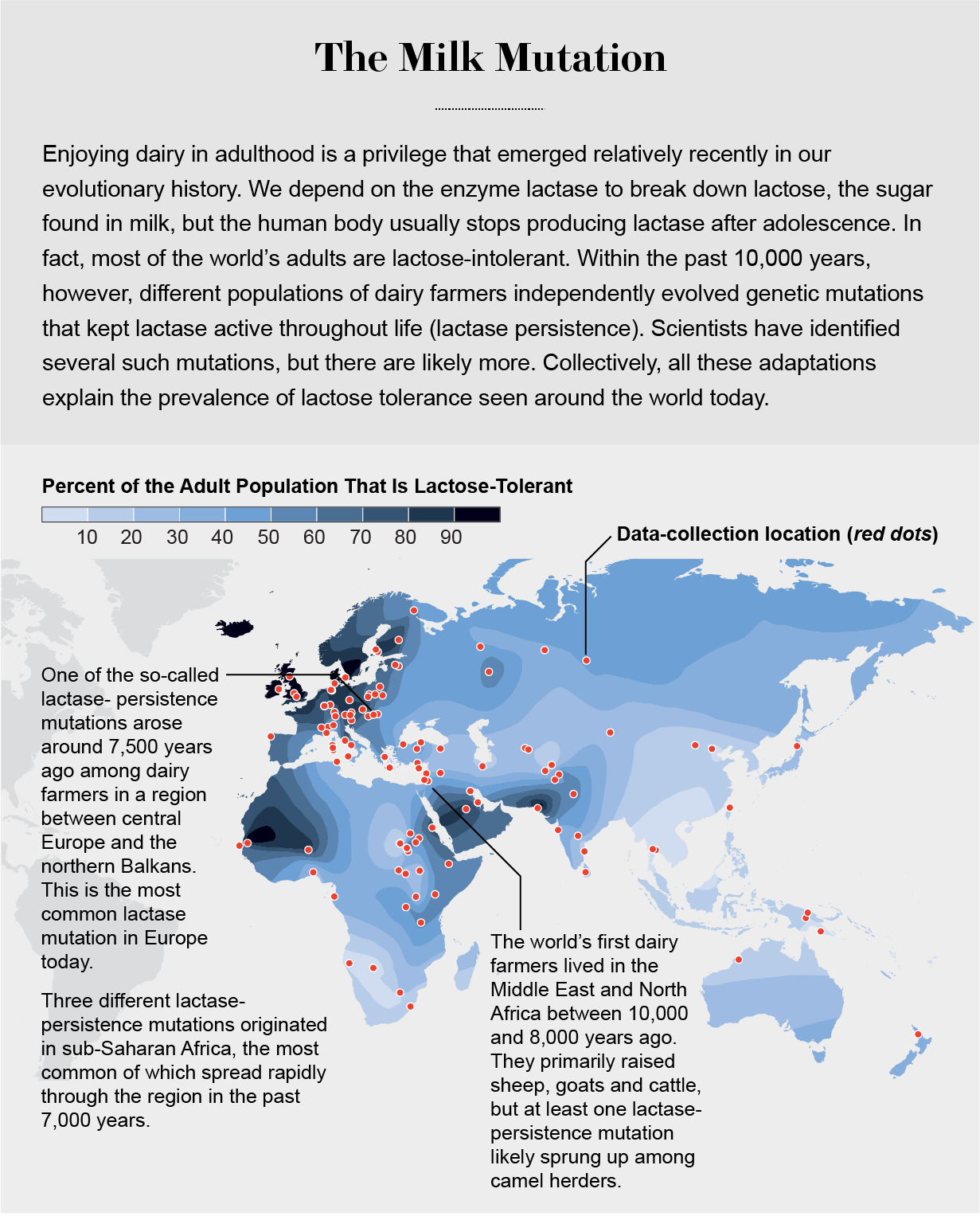
Another dietary adaptation is one of the best-studied examples of recent human evolution: lactose tolerance. Nearly everyone in the world is born with the ability to produce the enzyme lactase, which breaks down the milk sugar lactose and makes it easier to extract energy from milk—essential for the survival of a suckling child. Most people lose this ability by adulthood. At least five different times in our recent evolutionary past, as people started to discover dairy, a genetic mutation arose to lengthen the activity of the lactase gene. Three of the mutations originated in different parts of sub-Saharan Africa, where there is a long history of cattle herding. Another one of the five genetic tweaks is common in Arabia and seems to have sprung up in ancient populations of camel and goat herders.
The fifth and most common variant of the mutation that keeps the lactase gene turned on in adulthood is found today in human populations stretching from Ireland to India, with its highest frequencies across northern Europe. The mutation originated in a single individual 7,500 years ago (give or take a few thousand years). In 2011 scientists analyzed DNA recovered from Ötzi the Iceman, who was naturally mummified about 5,500 years ago in northern Italy. He did not have the lactose-tolerance mutation, a hint that it had not yet become common in this region thousands of years after its initial origin. In following years, researchers sequenced DNA extracted from the skeletons of farmers who lived in Europe more than 5,000 years ago. None carried the lactase mutation. Yet in the same region today, the lactase-persistence mutation occurs in hundreds of millions of people—more than 75 percent of the gene pool. This is not a paradox but the mathematical expectation of natural selection. A new mutation under selection grows exponentially, taking many generations to become common enough to notice in a population. But once it becomes common, its continued growth is very rapid, and ultimately it dominates.
Recent Traits
The Shallowness of Races
What is perhaps most extraordinary about our recent evolution is how many common physical features are completely new to human anatomy. The thick, straight black hair shared by most East Asians, for example, arose only within the past 30,000 years, thanks to a mutation in a gene called EDAR, which is crucial for orchestrating the early development of skin, hair, teeth and nails. That genetic variant traveled with early settlers of the Americas, all of whom share an evolutionary past with East Asians.
In fact, the overall evolutionary history of human skin, hair and eye pigmentation is surprisingly shallow. In the earliest stages of our evolution, all our ancestors had dark skin, hair and eyes. Since this initial state, dozens of genetic changes have lightened these features to some extent. A few of these changes are ancient variations present within Africa but more common elsewhere in the world. Most are new mutations that have emerged in one population or another: a change in a gene named TYRP1, for instance, that makes certain Solomon Islanders blond; the HERC2 mutation that results in blue eyes; changes to MC1R that cause red hairs to sprout instead of black ones; and a mutation in the SLC24A5 gene that lightens skin color and that is now found in up to 95 percent of Europeans. As in the case of lactase, ancient DNA is giving clear information about the antiquity of such mutations. Blue eyes seem to have appeared in people who lived more than 9,000 years ago, but the massive change to SLC24A5 is not found in the DNA of ancient skeletons from the same time period. Skin, hair and eye color evolved with stunning speed.
Variations in pigmentation are some of the most obvious differences among people and, in some ways, the easiest to study. Scientists have also investigated much odder and less evident features of human anatomy. Consider the variations of earwax. Most people in the world today have sticky earwax. In contrast, many East Asians have dry, flaky earwax that does not stick together. Anthropologists have known about this variation for more than 100 years, but geneticists did not uncover the cause until recently. Dry earwax results from a relatively new mutation to a gene called ABCC11. Only 30,000 to 20,000 years old, the mutation also affects the apocrine glands, which produce sweat. If you have stinky armpits and sticky earwax, chances are you have the original version of ABCC11. If you have dry earwax and a little less need for deodorant, you probably have the newer mutation.
A few thousand years before dry earwax first appeared among East Asians, another seemingly simple mutation started saving millions of Africans from a deadly disease. A gene called DARC produces a starchy molecule on the surface of red blood cells that mops up excess immune system molecules known as chemokines from the blood. About 45,000 years ago a mutation in DARC conferred remarkable resistance to Plasmodium vivax, one of the two most prevalent malaria parasites infecting humans today. Parasites of this species enter red blood cells through the DARC molecule encoded by the gene, so hindering the expression of DARC keeps the pathogens at bay. The absence of DARC also increases the amount of inflammation-causing chemokines circulating in the blood, which has in turn been linked to an increase in prostate cancer rates in Black men. Yet on the whole, the mutation was so successful that 95 percent of people living south of the Sahara now have it, whereas only 5 percent of Europeans and Asians do.
The Power of Random
We are accustomed to thinking about evolution as a process of “good” genes replacing “bad” ones, but the most recent phase of human adaptation is a testament to the power of randomness in evolution. Beneficial mutations do not automatically persist. It all depends on timing and on population size.
I first learned this lesson from anthropologist Frank Livingstone. The beginning of my training coincided with the end of his long career, during which he investigated the genetic basis for malaria resistance. More than 3,000 years ago in Africa and India, a mutation arose in the gene encoding the oxygen-transporting blood cell molecule known as hemoglobin. When people inherited two copies of this mutation—dubbed hemoglobin S—they developed sickle cell anemia, a disease in which unusually shaped blood cells clog vessels. Red cells are normally supple and flexible enough to squeeze through tiny capillaries, but the mutant blood cells were rigid and deformed into the characteristic “sickle” shape. As it turns out, changing the shape of red blood cells also thwarted the ability of the malaria parasite to infect those cells.
Another mutation that interested Livingstone was hemoglobin E. Common in Southeast Asia today, hemoglobin E confers substantial malaria resistance without the severe side effects of hemoglobin S. “Hemoglobin E seems like it would be a lot better to have than hemoglobin S,” I said in class one day. “Why didn't they get E in Africa?”
“It didn't happen there,” Livingstone said.
His reply stunned me. I had supposed natural selection to be the most powerful force in evolution's arsenal. Humans had lived with deadly Plasmodium falciparum malaria for thousands of years in Africa. Surely natural selection would have weeded out less helpful mutations and hit on the most successful one.
Livingstone went on to show how the previous existence of hemoglobin S in a population made it harder for hemoglobin E to invade. Malaria rips through a population full of only normal hemoglobin carriers, and a new mutation that provides a slight advantage can quickly become more common. Yet a population already supplied with the protective hemoglobin S mutation will have a lower mortality risk. Sickle cell carriers still face formidable risks, but hemoglobin E is less of a relative advantage in a population that already has this imperfect form of malaria resistance. Perversely, what matters is not only the luck of having the mutation but also when the mutation happens. A partial adaptation that has bad side effects can win, at least over the few thousand years humans have been adapting to malaria.
Since humans first began battling malaria, scores of different genetic changes have emerged that increase immunity to the disease, different ones in different places. Each started as a serendipitous mutation that managed to persist in a local population despite being very rare at first. Any one of those mutations was, individually, unlikely to last long enough to become established, but the huge and rapidly increasing population size of our ancestors gave them many more rolls of the dice. As human populations have spread into new parts of the world and grown larger, they have rapidly adapted to their new homes precisely because those populations were so big.
Our Evolutionary Future
Human populations continue to evolve today. Unlike the distant past, where we must infer the action of selection from its long-term effects on genes, today scientists can watch human evolution in action, often by studying trends in health and reproduction. Even as medical technology, sanitation and vaccines have greatly extended life spans, birth rates in many populations still vacillate.
In sub-Saharan Africa, women who have a certain variant of a gene called FLT1 and who are pregnant in the malarial season are slightly more likely to bear children than are pregnant women who lack the variant because the possessors have a lower risk that the placenta will be infected by malaria parasites. We do not yet understand how this gene reduces the risk of placental malaria, but the effect is profound and measurable.
Stephen Stearns of Yale University and his colleagues have examined years of records from long-term public health studies to see which traits may correlate with reproduction rates today. During the past 60 years, relatively short and heavy women in the U.S. who have low cholesterol counts had slightly more children on average than women who have the opposite traits. Why these traits have been related to family size is not yet clear.
Public health studies, such as U.K. Biobank, track the genotypes and lifetime health of hundreds of thousands of people. Such studies are being undertaken because the interactions of genes are complicated, and we need to examine thousands of outcomes to understand which genetic changes underlie human health. Tracing the ancestry of human mutations gives us tremendous power to observe evolution over hundreds of generations but can obscure the complex interactions of environment, survival and fertility that unfolded in the past. We see the long-term winners, such as lactase persistence, but may miss the short-term dynamics. Human populations are about to become the most intensively observed long-term experiment in evolutionary biology.
What will the future of human evolution look like? Across the past few thousand years, human evolution has taken a distinctive path in different populations yet has maintained surprising commonality. New adaptive mutations may have elbowed their way into human populations, but they have not muscled out the old versions of genes. Instead the old, “ancestral” versions of genes mostly have remained with us. Meanwhile millions of people are moving between nations every year, leading to an unprecedented rate of genetic exchanges and mixture.
With such a high rate of genetic mixing, it may seem reasonable to expect that additive traits—for example, pigmentation, where many different genes have independent effects on skin color—will become ever more blended in future human populations. Could we be looking at a human future where we are a homogeneous soup instead of a colorful stew of variability?
The answer is no. Many of the traits that differ between human populations are not additive. Even pigmentation is hardly so simple, as is readily seen in mixed populations in the U.S., Mexico and Brazil. Instead of a mass of clones, we are already starting to see a glorious riot of variations—dark-skinned, freckled blondes and striking combinations of green eyes and olive skin. Each of our descendants will be a living mosaic of human history.

