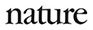Over the past six months, hundreds of millions of people around the world have rushed to follow in the footsteps of a 90-year-old British woman named Margaret Keenan.
At 6:30 a.m. on 8 December 2020, Keenan became the first person to receive a COVID-19 vaccine as part of a mass vaccination effort. Her shot was the culmination of a frenzied effort to develop vaccines safely and in record time. Now, more than 1.7 billion doses later (see ‘Global doses’), researchers are sifting through the data to address lingering questions about how well the vaccines work—and how they might shape the course of the coronavirus pandemic that has already taken more than 3.5 million lives.
“It’s absolutely astonishing that this has happened in such a short time—to me, it’s equivalent to putting a person on the Moon,” says paediatric infectious-disease specialist Cody Meissner at Tufts University School of Medicine and Tufts Children’s Hospital in Boston, Massachusetts. “This is going to change vaccinology forever.”
Nature looks at what lessons have emerged during the first six months of COVID-19 vaccinations, as well as what questions still linger. Overall, the vaccine results have been extremely promising—even better than many had hoped—but researchers have concerns about emerging variants and the potential for immune responses to wane.
How well do the vaccines work in the real world?
Danish epidemiologist Ida Moustsen-Helms was excited in February when she first saw how well the Pfizer–BioNTech vaccine was working in health-care workers and residents of long-term care facilities, who were the first to receive it in Denmark. A clinical trial in more than 40,000 people had already found the vaccine to be 95% effective in protecting recipients from symptomatic COVID-19. But Moustsen-Helms, who works at the Statens Serum Institut in Copenhagen, and her colleagues were among the first to test its effectiveness outside clinical trials, which can exclude some unhealthy individuals or those taking medicines that suppress immune responses.
The results showed it was 64% effective in long-term-care residents with a median age of 84, and 90% effective in health-care workers—which struck Moustsen-Helms as good news, given that immune responses in older people can be muted. But some Danish politicians were upset by the relatively low effectiveness in older recipients. “People were saying ‘how can this be true?’” she says. “Sometimes they forget that when you look at a trial result, those individuals included in trials are very different from people in the real world.”
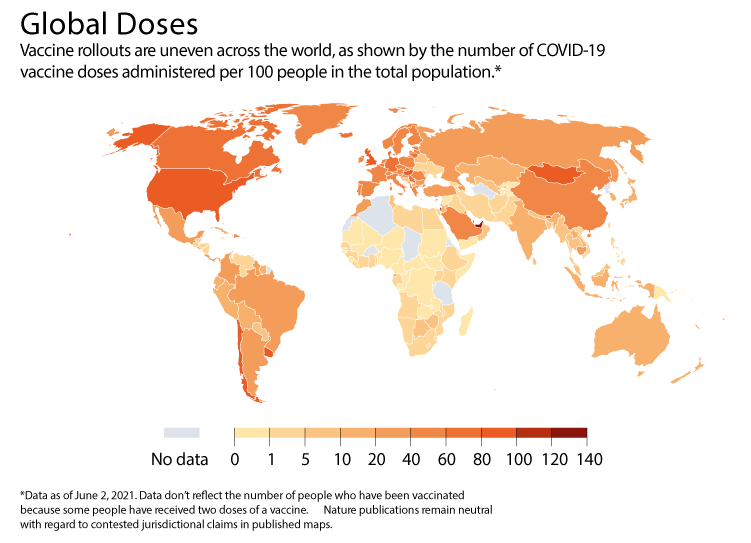
Since then, real-world data have come in from several countries (see 'Vaccination variation'), and much of the news has continued to be positive about how well vaccines perform in the general population. A nationwide vaccination campaign in Israel found the Pfizer–BioNTech vaccine, co-developed by Pfizer in New York City and BioNTech in Mainz, Germany, to be 95% effective against SARS-CoV-2 infection seven days or more after the second dose. The Gamaleya National Research Center of Epidemiology and Microbiology in Moscow and the Russian Direct Investment Fund announced that their Sputnik V vaccine has been 97% effective in almost 4 million people in Russia. And last month, London-based Public Health England reported that the Pfizer–BioNTech and Oxford–AstraZeneca vaccines are both 85–90% effective in preventing symptomatic disease after two doses. It cautioned, however, that it had low statistical confidence in the result for the Oxford–AstraZeneca jab, developed by the University of Oxford, UK, and AstraZeneca in Cambridge, UK.
Among older adults who received the Pfizer–BioNTech vaccine, Israel has seen 94% protection from SARS-CoV-2 infection in people over 85 years old. This is remarkably high for that age group, and considerably higher than Moustsen-Helms’s result of 64%, possibly in part because long-term-care residents are prone to be in poor health. Similarly, a UK study found that the Pfizer–BioNTech and Oxford–AstraZeneca vaccines were both 80% effective at preventing COVID-19 hospitalizations in people aged 70 or older. Studies are under way to see whether vaccine effectiveness can be boosted even more by mixing and matching vaccines, and early results have been promising. But the vaccines have already exceeded expectations, says Meissner, especially given how quickly they were developed—despite thorough safety testing in unusually large clinical trials—and the novel approaches they used. Some vaccines spend years in development, and still might not achieve this level of protection. “The efficacy of these vaccines is absolutely remarkable,” says Meissner.
At the other end of the age spectrum, Pfizer–BioNTech and Moderna in Cambridge, Massachusetts, have recently completed clinical trials of their vaccines in adolescents, showing 100% and 93% protection in those aged 12–15 and 12–17, respectively. Real-world data are not yet available. Meissner, who is an external adviser on vaccines to the US Food and Drug Administration, questions whether children under 12 should get the vaccines before the shots have received full regulatory approval—rather than an emergency-use authorization.
How effective are the vaccines against variants?
Soon after the triumph of Keenan’s first dose, the world had a fresh reason to worry. A SARS-CoV-2 variant identified in the United Kingdom seemed to be spreading unusually fast; a different variant first identified in South Africa carried worrisome mutations in the coronavirus spike protein that serves as the basis for most COVID-19 vaccines in use.
Since then, further ‘variants of concern’ have arrived in a steady parade, brandishing mutations that might boost the virus’s spread, or undermine the effectiveness of COVID-19 vaccines. “Uncontrolled outbreaks generate mutants,” says Jerome Kim, director-general of the International Vaccine Institute in Seoul.
Initial laboratory tests suggested that antibodies raised by the Pfizer–BioNTech vaccine were less effective against the B.1.351 variant identified in South Africa, but it was unclear how that would affect protection against disease. In May, researchers in Qatar published reassuring data showing that people who received two doses of the Pfizer–BioNTech vaccine were 75% less likely to develop COVID-19 from infection with B.1.351, and were almost completely protected from severe disease. “The big question right now is whether introduction of other variants could change the situation,” says study author and infectious-disease epidemiologist Laith Jamal Abu-Raddad at Weill Cornell Medicine–Qatar in Doha. “We are watching this on a daily basis, but we have optimism that maybe we have seen the worst.”
The Oxford–AstraZeneca vaccine did not fare as well in another test: in South Africa, a small clinical trial suggested that the vaccine did little to fend off infections of the B.1.351 variant that, by that point, was causing most infections there. As a result, the South African government made the difficult decision to sell its doses and await a different vaccine. It is now rolling out the vaccine produced by Johnson & Johnson in New Brunswick, New Jersey, which in one clinical trial was 64% effective at blocking moderate to severe COVID-19 in South Africa at a time when B.1.351 constituted more than 94% of the infections in the trial. And a vaccine made by Novavax in Gaithersburg, Maryland, which has not yet been authorized for emergency use, was 51% effective at preventing symptomatic COVID-19 among participants in South Africa who did not have HIV.
But Shabir Madhi, an immunologist at the University of the Witwatersrand in Johannesburg and a lead investigator on trials of the vaccine in South Africa, disagreed with the country’s decision not to use the Oxford–AstraZeneca vaccine. There was still hope that it could protect against severe disease and death, he says—a possibility that was not tested in the trial, which enrolled mostly young participants with a low risk of severe disease. Madhi notes that a later study in hamsters found that the vaccine prevented clinical disease caused by B.1.351.
The coronavirus SARS-CoV-2 has proved to be much more prone to mutations than researchers first thought, and more variants are emerging all the time. One variant of concern, called B.1.617.2, was first identified in India and is spreading rapidly in the United Kingdom, raising worries that it could be unusually transmissible. Public Health England has determined that two doses of either the Pfizer–BioNTech or the Oxford–AstraZeneca vaccines are 88% and 60% effective, respectively, at preventing symptomatic disease caused by this variant.
How long does protection against disease last?
Six months is not much time to collect data on how durable vaccine responses will be, but data could soon emerge from clinical-trial participants who had their first doses last July.
In the meantime, some researchers are looking to natural immunity as a guide. A study in more than 25,000 health-care workers in the United Kingdom found that a SARS-CoV-2 infection reduced the risk of catching the virus again by 84% for at least 7 months. And Abu-Raddad says an unpublished study in Qatar is finding about 90% protection against reinfection as much as a year after a bout of SARS-CoV-2. “It seems to suggest that immunity is really strong against this virus,” he says. “I’m optimistic that vaccine immunity is going to last more than a few months and longer than a year, hopefully.”
But Mehul Suthar, a viral immunologist at Emory University in Atlanta, Georgia, is concerned that vaccine-induced immunity will not be as durable as immunity from natural infection. Suthar says that he and his collaborators have found that antibody levels declined faster in those who were vaccinated with the Moderna vaccine than in those who had been infected by SARS-CoV-2. Antibodies are not the only determinant of immunity, he says, but the results worry him. “I’m a little concerned that the vaccines weren’t as robust in generating more durable antibody responses,” Suthar says. “When you factor in variants, to me it’s clear that we’re going to need a booster.”
How soon that booster is needed could depend in part on the rate at which antibody levels decline—they could drop precipitously or plateau at a low level. One modelling study estimates that low levels of antibodies will be enough to offer significant protection against severe disease. But Pfizer chief executive Albert Bourla has said that he expects a booster to be needed in about 8–12 months after the second dose of the Pfizer–BioNTech vaccine.
On 19 May, the UK government announced that it had funded a study of 7 different COVID-19 vaccines given as boosters at least 10–12 weeks after the second dose of an initial vaccine. Early findings are expected in September—in time to inform a booster programme aimed at protecting the most vulnerable groups over the UK winter. The US National Institutes of Health is also studying boosters in some study participants who received their first vaccine dose in an early clinical trial that began in March 2020.
Vaccine developers are now testing variant-specific boosters, too. Moderna has released preliminary results showing that a booster vaccine using a spike-protein sequence from the B.1.351 variant increased the concentration of antibodies that neutralize SARS-CoV-2, and particularly the B.1.351 variant.
Even if immunity does fade earlier than he hopes, Abu-Raddad is optimistic that it won’t disappear entirely. “If I would make a bet right now, I would say that even when people start losing their immunity against infection, they will not lose immunity against severe infections,” he says.
How much do vaccines block transmission?
Key clinical trials for currently authorized vaccines determined whether the inoculations could safely avert symptomatic disease in individuals. But blocking transmission of the virus is also crucial for ending a pandemic, and most of those clinical trials did not track asymptomatic infections that could fuel the virus’s spread.
Researchers have been trying to fill this gap, and, so far, the data look promising. Results announced by Johnson & Johnson from clinical trials suggest that its vaccine is 74% effective against asymptomatic infections. Researchers studying deployment of the Pfizer–BioNTech vaccine in Israel have also reported that vaccination reduces the amount of virus found in infected individuals by up to 4.5-fold, suggesting that they could be less likely to shed that virus into the environment, where it might infect someone else.
And a study by Public Health England has found that even a single dose of either the Pfizer–BioNTech or Oxford–AstraZeneca vaccine reduced the spread of disease from infected individuals to household members by up to 50%. “It’s likely that all the vaccines have some similar effect,” says Michael Weekes, a viral immunologist at the University of Cambridge, UK. “Overall, it’s quite an optimistic picture.”
But, faced with incomplete data, these studies must often rely on inference to draw conclusions—assuming, for example, that lower viral load translates to reduced transmission, says Susan Little, an infectious-disease specialist at the University of California, San Diego. Little is an investigator on an ambitious trial spread across more than 30 higher-education institutions in the United States to determine how often vaccinated people infect others. The trial will randomize students so they either receive the Moderna vaccine or delay vaccination by four months. Researchers will test participants daily for infection; their close contacts will take coronavirus tests twice a week.
Little and her colleagues are looking for high-quality data to back up important decisions to come. “As people are starting to go back to work, at a policy level, should vaccination be required for schools, places of employment, public transport?” she asks. “Do vaccinated individuals need to wear masks or social distance?” On 13 May, the US Centers for Disease Control and Prevention revised its guidelines on masking, saying that fully vaccinated people could go without masks in some public settings.
But Little says widespread vaccine availability in the United States has left the study struggling to enrol participants. And the spread of viral variants could complicate the picture still more, says Kim. If vaccines are less able to decrease the viral load in individuals infected with a variant, they might also be less able to block transmission, he cautions. “Transmission is a really hard one,” he says. “And an unknown variable here is how the variants will affect this.”
What have scientists learnt about safety?
The speed at which countries have rolled out COVID-19 vaccines is unparalleled—and the same can be said of the surveillance systems put in place to monitor vaccine safety.
Clinical trials of some vaccines involved more than 40,000 participants, and yielded few signs of side effects beyond those often seen after vaccination, including injection-site soreness, fever and nausea. “We generally say that no vaccine is 100% safe,” says Meissner. “But the safety of these vaccines is remarkable.”
Shortly after inoculations with the Pfizer–BioNTech vaccine began, a few regions reported cases of a severe allergic reaction called anaphylaxis. But further study showed that the risk of this condition—which can be treated at the vaccination centre—is not much higher for the Moderna and Pfizer–BioNTech jabs than for other vaccines, says Meissner. For Pfizer–BioNTech, the risk is about 4.7 cases per 1 million doses; the risk of anaphylaxis from any vaccination is estimated at 1.3 in a million.

More concerning has been the very rare occurrence of a blood-clotting syndrome in recipients of the Oxford–AstraZeneca and Johnson & Johnson vaccines. First reported in Europe and linked to vaccination with the Oxford–AstraZeneca vaccine, hallmarks of the syndrome include blood clots in unusual places—particularly in the brain and abdomen—coupled with depletion of clot-promoting cell fragments called platelets. The condition can be fatal, but regulators have repeatedly determined that the risk posed by COVID-19 is greater for many people than is the risk of developing the clotting syndrome. The European Medicines Agency has concluded that it occurs in about one in 100,000 vaccine recipients.
Researchers are still racing to determine how the vaccine could cause the syndrome. But the subsequent US discovery of similar cases among recipients of the Johnson & Johnson vaccine—although at a frequency of only about 3.5 per million people—has led to speculation that the condition might be linked to the disabled adenoviruses used in the vaccines to shuttle the coronavirus spike gene into cells.
Since the syndrome was discovered, the United Kingdom has advised that people under the age of 40 receive a different vaccine, given their very low risk of complications from SARS-CoV-2 infection. The United States has resumed vaccinations with the Johnson & Johnson vaccine after pausing it in response to the reports. But in Denmark, the Oxford–AstraZeneca vaccine was discontinued in April, and those who have already received one dose have been advised to have an mRNA vaccine from Pfizer–BioNTech or Moderna as their second dose.
Meanwhile, surveys have suggested that the debate over the safety of these vaccines was enough to damage public confidence in them. “What defines a safe vaccine?” says Meissner. “One out of a hundred thousand may seem very safe for one person; another person says ‘One in a million? What if that’s me?’”
Israel’s Ministry of Health is now evaluating a possible link between the Pfizer–BioNTech vaccine and reports of heart inflammation, a condition called myocarditis. So far, most cases have been mild and have occurred in men aged between 16 and 19.
What impact have the vaccines had on the course of the pandemic?
Several countries with high vaccination rates—including Israel and the United Kingdom—have seen precipitous declines in deaths and hospitalizations from COVID-19. Public Health England has calculated that the vaccines have saved 13,000 lives among those aged 60 and over4. The United Kingdom has fully vaccinated more than one-third of its population.
But these countries have conducted their vaccination campaigns while under strict social-distancing measures. Chile, by contrast, rolled back its distancing requirements early this year as it embarked on an aggressive vaccination campaign. By April, its intensive-care wards were overflowing with COVID-19 patients, despite the country having one of the world’s highest vaccination rates.
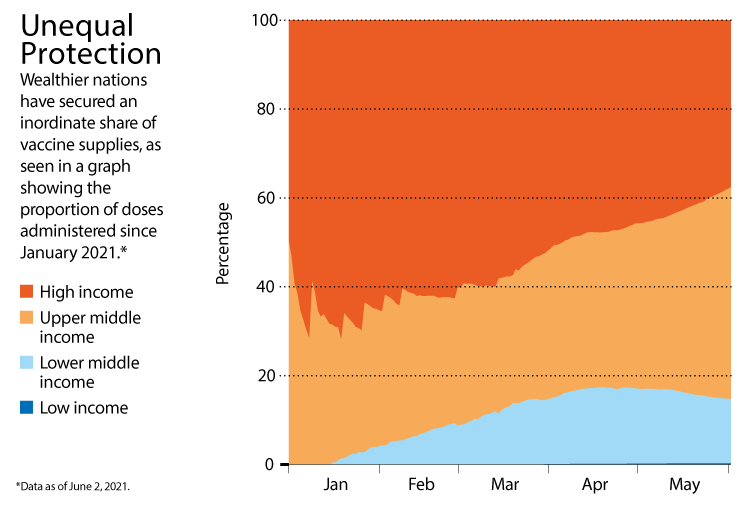
Once vaccines have reached a wide swathe of the population, however, it might be possible to ease lockdowns and social-distancing restrictions. Israel’s rates of infection, for example, have remained low after it gradually relaxed most restrictions once about half of its adult population had been vaccinated. Infections are also falling in the United States as the proportion of fully vaccinated adults there surpasses 40% (see ‘Unequal protection’).
But the Seychelles, the most vaccinated country in the world (with a population of less than 100,000), experienced a surge in infections—although relatively few deaths—as it reached a level of more than 60% adult vaccination in early May.
For now, it’s unclear what has driven that outbreak and whether coronavirus variants could be to blame, says Kim. But it pays to ease restrictions slowly, he says, even once a country has achieved a high level of vaccination. “It’s probably wise to remember that every time we saw the numbers going down and we were relieved and relaxed, they came back again,” says Kim. “That’s the cautionary tale in all of this.”
And for much of the world—particularly low- and middle-income countries—limited supplies mean that vaccines will probably have little impact on the course of the pandemic this year. Madhi says that he does not expect the current roll-out in South Africa to do much to protect it from the impending third surge there: by the time all people over the age of 60 have been offered their first dose at the end of June, he expects social distancing and other measures to have already brought the country’s burgeoning infection numbers down. And in India, a combination of low vaccination rates, aggressive variants and widespread social interaction are thought to have led to its tragic and overwhelming COVID-19 outbreak.
Whereas some wealthy countries were able to pre-order large amounts of vaccine, many low- and middle-income countries have had to make do with less. The World Health Organization’s target is to vaccinate 20% of the population in those countries by the end of this year. “This is not going to be the main exit strategy for them this year,” says Mark Jit, an infectious-disease modeller at the London School of Hygiene & Tropical Medicine. “Maybe in 2022, when the supply is less constrained.” Instead, such countries might need to rely heavily on social distancing, mask wearing and test-and-trace programmes.
And even in countries with higher vaccination rates, the once-glittering hope of achieving herd immunity—when enough immunity exists in the population to prevent disease spread—has faded, says Kim. “Now with widespread generation of these variants and continued uncontrolled outbreaks, that’s looking less likely,” he says. “And the impact of the pandemic will continue to be felt until vaccination can be accomplished not only in high-income but low- and middle-income countries.”
This article is reproduced with permission and was first published on June 4 2021.