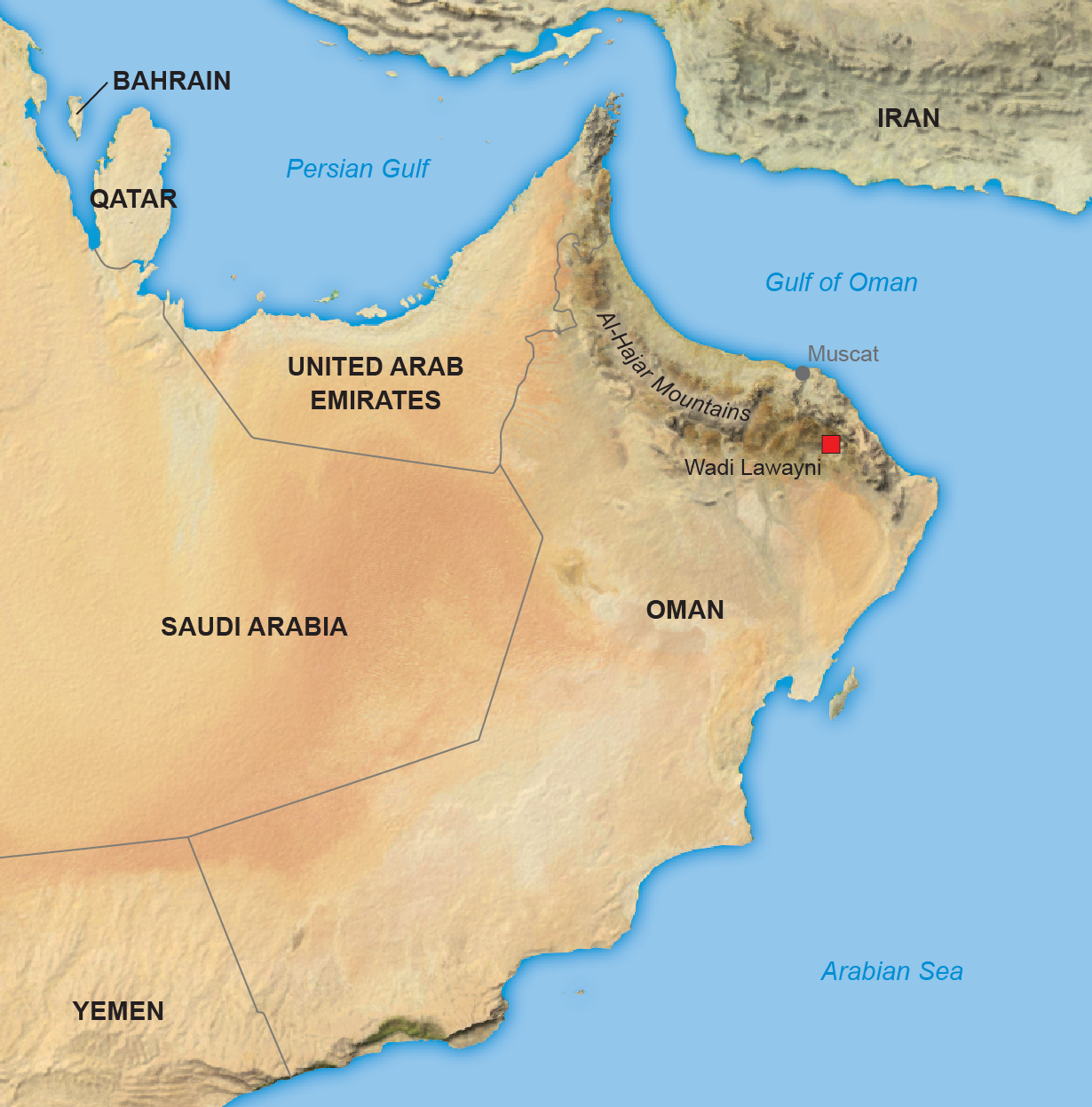
Wadi Lawayni is a remote desert valley in the interior Al-Hajar Mountains of Oman, east of Saudi Arabia. A visitor gets there by following a lonely dirt road that dwindles to tire tracks running through a gravelly wash. Groundwater in this region occasionally surfaces in small pools that have a bluish tint—saturated with alkaline salts and sometimes so full of hydrogen gas that the liquid fizzes like champagne when it’s raised out of a well.
The valley, sparsely dotted with thorny shrubs, is ringed by worn pinnacles of faded brown stone that rise hundreds of meters into the air. The rock is an anomaly of minerals that are chemically unstable on Earth’s surface. It may have formed dozens of kilometers below the surface, within the mantle—the middle layer of our planet, which humans have never directly seen—far deeper than any oil well or diamond mine. The rock was shoved to the surface through an accident of plate tectonics around 80 million years ago, and now that it is exposed to the elements, it is undergoing a smoldering, flatulent geochemical decay.
Peter Kelemen thinks this geologic oddity might help humans change the course of the climate emergency. He introduced this vision to me one afternoon in January 2018 as we sat in camp chairs in Wadi Lawayni in the tattered shade of a scraggly acacia tree. A hundred meters away, under a canopy, was a makeshift outdoor laboratory with tables, chemicals and a specialized scanner for examining rock samples. Now 65, Kelemen is a geologist at the Lamont-Doherty Earth Observatory at Columbia University, with cropped gray hair and skin tanned by decades of working outside. Leathery dollops of camel dung were strewn in the gravel at our feet. Kelemen motioned to the wall of rock behind us, made of brownish, weathered mantle rock called peridotite. When rain percolates through cracks in the rock, it brings dissolved oxygen and carbon dioxide from the air. The water and gases react with the rock, forming solid veins of new minerals that dig, like tree roots, ever deeper into the stone. The rock was crisscrossed with these creamy-white veins. Kelemen pointed to one a centimeter across composed of magnesium carbonate. “That’s about 50 percent CO2,” he said. When I tapped it with a pebble, it emitted a glassy clang.
Kelemen and his colleagues estimate that Oman’s exposed mantle rocks are absorbing and petrifying up to 100,000 metric tons of CO2 every year. That’s roughly one gram of the greenhouse gas per cubic meter of stone. “If you [enhance] that by a factor of a million”—something Kelemen thinks is doable with a bit of engineering—“then you end up with a billion tons of CO2 per cubic kilometer of rock per year,” he says. And Oman, with about 15,000 cubic kilometers of the rock, has plenty of capacity. Kelemen’s plan involves accelerating the natural reactions by drilling several kilometers down, to where the rocks are hotter, and pumping in seawater saturated with CO2 drawn from the air.

Similar outcrops emerge from Earth’s surface in Alaska, Canada, California, New Zealand, Japan, and other places. Kelemen estimates the worldwide storage capacity of these rocks, including Oman’s, as 60 trillion to 600 trillion tons of CO2—roughly 25 to 250 times the amount that humans have added to the atmosphere since 1850. Kelemen says exploiting that stony repository could have a huge impact. A 2019 report by the Intergovernmental Panel on Climate Change concluded that global warming cannot be limited to 1.5 degrees Celsius—a level generally thought to avoid catastrophic impacts—unless humans somehow remove between 100 billion and one trillion tons of CO2 from the atmosphere by 2100. If the process started by 2050, that would mean drawing down two billion to 20 billion tons of CO2 every year.
For this vision to materialize, humans would have to build an extensive, global infrastructure of machines that pull CO2 from the atmosphere and inject it down wells drilled into mantle rock—a kind of mirror image of the infrastructure that currently extracts fossil fuels, which when burned send CO2 into the air. Kelemen sees Oman as a bustling center of this vast new industry.
Whether this counterinfrastructure could work will depend on investigations unfolding in Oman. While we chatted under the tree, Kelemen’s team of scientists was getting ready to drill down into Wadi Lawayni’s floor and extract 400 meters of rock core to study the chemical reactions happening below our feet. A backhoe rumbled in the distance, excavating a pit in preparation for drilling.
The findings, published in 2019 and 2020, revealed a clear path for how humans could enhance the reactions. In late May of this year a new team of workers was scheduled to arrive in Wadi Lawayni to conduct the world’s first test of injecting and mineralizing CO2 deep in mantle rock. If that experiment succeeds, it could be the first step toward transforming Oman, or even the greater Arabian Peninsula, into a major industrial center for managing the climate emergency.
Fast Reactions
Scientists have spoken for decades about counterbalancing greenhouse gas emissions by capturing airborne CO2 and pumping it into the ground. But an increasing number of studies now indicate that the need for such “negative carbon emissions” has become urgent. Scientists have proposed various strategies. Replanting forests or fertilizing the ocean would, respectively, increase the growth of trees or phytoplankton that naturally absorb CO2 through photosynthesis. Improved farmland management would allow more of the CO2 absorbed by crops to remain in the soil after the plants are harvested. “Carbon capture” equipment could filter CO2 from the smokestacks of power plants or factories, and thousands of “direct-air capture” machines around the world could pull it from the atmosphere night and day.
Captured CO2 has to be permanently sealed away. A few operations have tried. At the Sleipner gas field off Norway’s coast, CO2 that comes up with the natural gas is injected back into sedimentary rocks—grainy deposits such as sandstone—in the reservoir a kilometer below the seafloor. This project, begun in 1996, stores roughly a million tons of CO2 a year. The issue with such operations is that the CO2 barely reacts with sedimentary rocks. It mostly percolates amid the rock’s pores, leading some scientists to worry that it could gradually leak back out.
Kelemen spent the 1990s pursuing a different line of scientific research, camping for weeks in Oman’s remote valleys, called wadis, mapping fossilized vents that once carried magma from deeper, hotter layers of the mantle up toward the surface, where it solidified into rock known as basalt—a hard, dense, dark stone that also forms much of the ocean’s crust. But when Kelemen moved in 2004 from the Woods Hole Oceanographic Institution in Massachusetts to Lamont-Doherty, he met geochemist Juerg M. Matter (now at the University of Southampton in England) and physicist Klaus S. Lackner (now director of the Center for Negative Carbon Emissions at Arizona State University). Lackner and Matter were exploring whether CO2 could be injected into rocks high in magnesium and calcium, which are more chemically reactive than sedimentary rocks and would readily convert the gas into solid minerals—a process called mineral carbonation.


Oman’s mantle peridotite rocks contain high levels of magnesium and calcium in two abundant minerals: olivine and pyroxene. These rocks are shot through with carbonate veins, so they have clearly absorbed CO2 in the past. But some researchers had assumed it happened over millions of years. Kelemen had never thought much about carbon disposal, yet he was skeptical about the reactions being so slow. While working in Oman, he had often walked past an alkaline spring in a valley called Khafifah, where water burbling from the ground was so saturated in calcium that it continuously reacted with CO2 in the air, forming a smooth, pearly, paper-thin coat of the carbonate mineral calcite on the surface of the pools. Kelemen noticed that when the calcite film was shattered by wind or rain, it was replaced by a new film within 24 hours. “For a geologist, a thing that happens in a day—that’s supersonic,” he says.
This fast reaction at the surface made Kelemen wonder whether veins might also be forming underground more quickly than people thought. When he traveled again to Oman in 2007, he and his students collected chunks of carbonate veins. Back home, they had the minerals dated. “I thought those veins would be 90 million years old,” Kelemen admits. “They were all less than 50,000 years.” Some were only 6,000 years old. Oman’s mantle rocks had not just absorbed CO2 in the distant past; they were still doing it now, perhaps 10,000 times faster than Kelemen had imagined.
During another trip, in 2008, Kelemen and Matter calculated that the minerals accounted for about 1 percent of the rock volume near the surface. That would mean the entire region was naturally solidifying 10,000 to 100,000 tons of CO2 a year—roughly equal to the annual emissions of 2,000 to 20,000 American automobiles. This amount wouldn’t make a dent in climate change, but it caused them to consider whether they could accelerate the process enough to make a worldwide difference.
The two researchers returned to Oman every year for the next four years. They sampled water from wells to trace the chemical reactions that occur as it travels underground. The results suggested that as rain soaks into cracks in the ground, CO2 dissolved in the rainwater pairs up with magnesium atoms, forming magnesium carbonate veins, until the small amount of gas in the water quickly runs out. Meanwhile calcium from the same mantle peridotite rock dissolves and accumulates in the water as it migrates. They thought that this calcium-rich water eventually reemerged at springs such as the one in Khafifah. There it reacts with CO2 in the air to form the calcite films that Kelemen had seen, as well as forming vast stepping-stone terraces of a calcite rock called travertine, which are scattered across the region.
Kelemen and Matter still didn’t know how much humans could speed up this process. It would depend on how deeply and quickly the water circulated. To answer that question, they needed to get below the surface.
Deep Water
On a warm, cloudless afternoon in January 2018, I watched as Kelemen and Matter got a crucial look inside the rocks of Wadi Lawayni. Eight camels chewing on shrubs paid no attention to the grinding roar of the drill mounted on the back of a bulky work vehicle, spinning as it bored into the valley floor.
A cable had already hoisted nine meters of core out of the hole. The core sections, each a couple of meters long and as wide as a baseball bat, were laid out in order on folding tables as half a dozen scientists inspected them. “There’s a lot of action in the first few meters,” Kelemen said as he moved with purpose from table to table. The color of the rock changed notably with even these relatively small changes in depth.
When the mantle rocks were still deep underground, they would have been dark green because of the magnesium- and calcium-rich minerals olivine and pyroxene formed at temperatures above 1,300 degrees C, in the complete absence of oxygen, water and CO2. But by the time tectonics and erosion brought the rocks to the surface, the minerals had undergone waves of chemical reactions. The top several meters of rock were tinged in shades of orange, showing that in the layers closest to the surface, oxygen, carried by water, had bonded with iron in the minerals, essentially rusting the rock. A few meters down those colors disappeared, meaning the dissolved oxygen had been exhausted in the water that had percolated that deep. By this point, the gray rock was perfused with countless, hair-thin turquoise-colored veins—a mineral called serpentine, which forms as water molecules attach to magnesium and iron atoms. (The process produces the hydrogen gas that fizzes from groundwater.)
Crisscrossing that background were white carbonate veins, which had formed as CO2 latched on to magnesium and calcium. Those veins started out about finger width, but by 10 meters down they were rare and thin, indicating that water had also lost its CO2 as it seeped downward.
As drilling continued over the coming days, workers packed the cores into crates to make way for dozens of new segments that crowded the tables, creating a veritable flea market of stone cylinders. The rocks from 400 meters deep were still permeated with fine serpentine veins, confirming that water had percolated at least that far down.
Analysis continued at the scientists’ labs over the next three years to determine how quickly the rocks were reacting with CO2 and water. In 2020 and early 2021 I spoke several times with Matter, and he was struck by one pattern seen in all the cores: “You find absolutely no carbonate minerals in veins or fractures below a maximum of 100 meters,” he said. For whatever reason, CO2 was not getting any deeper into the rocks.
Recent analyses published by the team suggest why this may be. In a 2019 paper, Kelemen and his colleagues, including Matter and Matter’s former student Amelia Paukert Vankeuren, now at California State University, Sacramento, estimated that groundwater in the upper 50 meters of the boreholes had been there for four to 40 years; it had seeped in from rainfall. But water in the rocks below that had been underground for at least 20,000 years. For a paper published in 2020, Matter and his collaborator Gérard Lods of the University of Montpellier in France measured how readily water moves through the rock by pumping water between two deep boreholes 15 meters apart. They found that water moved relatively easily at spots in the upper 100 meters, but below that the permeability dropped 1,000-fold.
Taken together, these observations show that the rate of mineral carbonation in Oman is limited by a major bottleneck: rainwater simply does not go deeper than about 100 meters. And Oman’s mantle rocks are, on average, about three kilometers thick. “It tells us that there’s huge potential for carbonation deeper down,” Matter says—if water can somehow get down there and circulate rapidly through the rocks so that it provides a steady supply of CO2.
To overcome this bottleneck, direct-air capture machines, which have fans that pull air through chemical absorbents, would remove CO2 from the air and concentrate it. Other equipment would pressurize the gas and send it down a borehole. At 1,000 to 3,000 meters down, the gas would be mixed with water (injected through a separate pipe), and the water with dissolved CO2 would be released into the surrounding mantle rocks. The water would seep through the rock’s pores, eventually reaching a second hole as much as 1,000 meters away that would act as a return chimney. The water, depleted of CO2, would rise back to the surface, where more gas could be concentrated in it again.
Rock temperatures three kilometers down are about 100 degrees C. That heat would accelerate the reactions. Additional heat generated by the reactions themselves would help drive the circulation of warmed water back up the chimneys.
In 2020 Kelemen and Paukert Vankeuren published calculations suggesting that pumping water containing mildly elevated concentrations of CO2 down to three kilometers could accelerate mineralization by many thousands of times. At that rate a single injection well could capture up to 50,000 tons of CO2 a year—similar to the amount of the gas being absorbed naturally in all of Oman—under an area of ground about the size of nine soccer fields. Over 10 years that well could capture half a million tons of CO2.
The scientists extracting cores in Wadi Lawayni did not attempt to inject CO2 into mantle rocks. But a few years earlier scientists in Iceland had tried injecting CO2 into a different type of rock that is chemically similar to the mantle. That successful project set the stage for what is now about to happen in Oman.
Frack Factor
Hundreds of kilometers underneath the North Atlantic Ocean, between Greenland and Norway, lies a hotspot in the mantle. Heat rising from Earth’s core softens the rock. This “partial melt” magma rises buoyantly through cracks up to the seafloor. For 50 million years this magma solidified into basalt—a rock that is derived from the mantle and is one of the major components of our planet’s crust. This plateau of basalt rose higher and higher above the seafloor until it emerged from the ocean, forming modern-day Iceland. The gray-black rock is dense, peppered with tiny bubbles. It contains less magnesium and calcium than its parent rock but still more than most rocks on Earth’s surface.
By 2005 Matter, Lackner and Wallace Broecker of Lamont-Doherty were convinced that these basalts provided a good opportunity for mineralizing CO2. Broecker (who died in 2019) collaborated with Reykjavik Energy to initiate a CO2-injection experiment called Carbfix at Iceland’s Hellisheidi geothermal power plant. Starting in 2012, machines separated CO2 and hydrogen sulfide gas—natural products of the geothermal sites—from the plant’s exhaust and injected them through wells 400 to 800 meters back down into the basalt.


Over eight months engineers injected about 250 tons of CO2. Monitoring at nearby wells showed that 95 percent of it was locked into carbonate minerals within two years. The project has operated ever since, storing roughly 10,000 tons of CO2 per year. In 2019 Carbfix was spun off as an independent company with the goal of locking a billion tons of CO2 into basalt by 2030.
Matter, who helped to lead the experiment, sees its results as a major validation. At the beginning, he says, “the carbon capture community thought we were crazy” because basalts were not thought to be porous enough to circulate water through. Since then, another team at Pacific Northwest National Laboratory in Richland, Wash., has also mineralized CO2 in basaltic rocks—the Wallula Basalt Pilot Demonstration.
Mantle rocks could be more potent than basalts because they contain three times as much reactive magnesium and calcium. One ton of mantle peridotite could solidify up to 500 kilograms of CO2, compared with about 170 kilograms for a ton of basalt.
But not everyone thinks that mantle rocks, or even basalts, are the perfect solution. Christopher Zahasky, a hydrogeologist at the University of Wisconsin–Madison, says that even though CO2 injected into sedimentary rocks can migrate, its storage is still secure because powerful capillary forces trap it in the tiny spaces between mineral grains. Even if rock above fractures, the gas is unlikely to leak out.
Zahasky still sees an important advantage to storing CO2 in basalts and mantle rocks, though. “It’s just easier to sell and explain to people,” he says—an important consideration given that large-scale projects are unlikely to happen without strong public support. And in some regions such as Oman, India and the U.S. Pacific Northwest, basalts or mantle rocks may be more plentiful than suitable sedimentary deposits. To solve the carbon problem, Zahasky notes, “we really need to throw everything at the wall.”
The challenge with mantle rocks, Zahasky says, is that they have far less pore space than sedimentary rocks. “You need more wells to distribute fluids within the subsurface more evenly,” he says. Kelemen has gnawed on this problem for years. He thinks there’s a solution: if injection is done correctly, the chemical reactions themselves could fracture the rocks, allowing water to move through.
When I was in Oman, Kelemen led me down a narrow wash. We stopped beside a rounded hunk of rock the size of a car, riddled with carbonate veins. Brick-sized blocks of rock that once fit snugly together were now tilted and pushed haphazardly apart by the intervening veins—like a ruined building in which the mortar between the bricks has expanded out of proportion. “When I look at this outcrop, I can almost hear it exploding,” Kelemen said.
This figurative “explosion” happened in slow motion while the rocks were still underground. When CO2 attaches to magnesium or calcium to form carbonate minerals, it adds mass. The new material occupies 20 to 60 percent more volume than the prior minerals did. Kelemen’s modeling suggests that these carbonate minerals can exert up to 2,900 atmospheres of pressure on the surrounding rock as they grow, pushing the rock apart. The chemical conversion of mantle rocks, Kelemen says, should naturally fracture them—driving cracks ever deeper and wider, exposing new reactive surfaces and allowing more water and CO2 to trickle in.
Matter and Robert Sohn, a geophysicist at Woods Hole, uncovered evidence of this fracking during two trips to Wadi Lawayni in 2019 and 2020. They lowered hydrophones into several water-filled boreholes that remained after drilling and placed seismometers around the holes. Over the course of a month, they recorded hundreds of microearthquakes that were far fainter than anything a person could feel. “If you have this reaction-driven cracking, it’s going to generate these very distinctive signals,” Sohn says. The data, he says, “were full of those signals.” He cautions that the results are consistent with reaction-driven cracking but don’t yet prove it.
Even if engineers could figure out how to harness expansion and cracking to their advantage, they would need to consider unintended consequences. Ballpark estimates suggest that trapping a billion tons of CO2 in carbonate minerals could potentially increase the volume of the rock by up to a tenth of a cubic kilometer, equal to about 35 Empire State Buildings. If that expansion were distributed in the rocks underlying 300 square kilometers of land—as it would be in one of Kelemen’s scenarios—then mineralizing a billion tons of CO2 a year could conceivably cause the ground to rise by up to 30 centimeters a year.
Injecting just a million tons of CO2 a year over 300 square kilometers would lead to less than a millimeter of ground rise a year—less than what naturally occurs in many areas from tectonic forces. The expansion becomes problematic only at truly vast scales of injection. Kelemen thinks that to reckon with the issue, any gigaton-scale injection in Oman should occur near the shores of the Gulf of Oman, where engineers could drill diagonally into mantle rocks that sit below the shallow seafloor. Any bulging would probably occur on the seafloor, where it would likely be benign. And the site would obviously provide plentiful seawater to carry concentrated CO2, important because groundwater tends to be scarce in this desert nation.
Clearly, questions need to be answered before mantle rocks can start making a dent in CO2 emissions. A trial to address those questions is beginning.
Conquering Cost
An Oman-based company named 44.01 (after the average molecular weight of CO2) has received government approval to run the world’s first pilot test of mineral carbonation in mantle rocks. The company was to begin moving equipment into Wadi Lawayni in May or June 2021. A few weeks later 44.01 would start injecting freshwater containing CO2 and an inert tracer chemical into a borehole a short distance from the one I observed in 2018. Researchers would monitor the levels of tracer, CO2 and dissolved minerals in a second borehole around 100 meters away to determine how quickly water is traveling through the intervening rock and how much CO2 is being stripped from it. Kelemen and Matter are advising the company. If this experiment shows that CO2 is mineralizing quickly—which 44.01’s founder, Talal Hasan, should know within about four months—the company plans to begin its first commercial injection operation in 2022. It would use freshwater or possibly treated wastewater to carry 10,000 tons of the gas a year down a single well, with hopes of eventually expanding to 100,000 tons a year. The company also plans to initiate a second pilot test closer to the coast, using seawater.
Hasan, an entrepreneur, envisions 44.01 as a mineral carbonation company that will sell its services to firms such as Switzerland-based Climeworks or British Columbia–based Carbon Engineering, which would run their direct-air capture machines in Oman. Carbon dioxide emitted into the air from anywhere on Earth wafts around the planet, so the gas can be captured and disposed of wherever it’s convenient. Oman could become a major global center.
Hasan thinks 44.01 could one day mineralize 1.3 billion tons of CO2 annually in Oman’s mantle formation. That quantity would make a meaningful dent in the two billion to 20 billion tons of CO2 that humans need to remove from the air every year to stay within 1.5 degrees C of warming. Currently 44.01 is the only company trying to inject CO2 into mantle rocks, but a 2019 report by the National Academy of Sciences suggests that similar mantle formations around the world could lock away upward of 10 billion tons a year. Operations in basalt, such as the one by Carbfix, would add further capacity.
Sequestering a billion tons of CO2 a year in Oman would require massive infrastructure. Kelemen has calculated that if the gas were concentrated to 440 times what naturally occurs in seawater—which can readily be done by today’s air-capture machines—5,000 injection wells would be needed. Together they would pump a combined 23 cubic kilometers of water a year—about 4 percent of the flow of the Mississippi River. The scale of such an operation might sound shocking, but the climate emergency requires a tremendous intervention. And this operation would still be quite small compared with the infrastructure that extracts fossil fuels. There are more than a million oil and gas wells in the U.S. alone. Of course, it would be up to humanity to use the opportunity to switch to renewable energy rather than as a license to emit even more carbon.
Cost will be pivotal. Carbfix in Iceland is mineralizing CO2 for about $25 a ton (44.01 is not releasing any official cost estimates). That falls within the price ranges of strategies such as reforestation and crop soil management, which store carbon less permanently, according to a 2018 report by an international group of scientists, published in Environmental Research Letters.
The real challenge is capturing and concentrating the CO2 before it’s injected. Kelemen’s collaborator Jennifer Wilcox, who is serving as the U.S. principal deputy assistant secretary for fossil energy, and her Ph.D. student Noah McQueen of the University of Pennsylvania have developed a framework for estimating the combined cost of capturing and compressing CO2, including workers’ salaries and expenses for building and maintaining the hardware over 20 years. Their numbers suggest that the cost would be roughly $120 to $220 per ton of CO2 removed from the atmosphere. Direct-air capture requires a lot of energy, and “if you choose to use fossil [fuels],” Wilcox says, “you have to think about the cost of managing the additional carbon” produced. The technology is still young, says Ajay Gambhir, a climate economist at Imperial College London, and innovation could bring its costs down. If the machinery follows the same trajectory that wind turbines have over the past decade, it could cost about a quarter of the rate today. But “we don’t really know until it’s scaled up,” Gambhir says.
It is unlikely that many emitters will pay for air capture and mineral carbonation until governments place a price on greenhouse gas emissions. Gregory Nemet, an energy policy researcher at the University of Wisconsin–Madison, says that although current carbon taxes imposed by industrialized countries are generally under $50 per ton of CO2, a carbon fuel standard in California is prompting companies to spend up to $200 a ton on carbon credits, with prices likely to increase over time. That figure provides an opening for a mineralization company such as 44.01 working in partnership with a direct-air capture company to start a small joint operation. As more governments institute carbon pricing, the demand for these services could grow. “They don’t have to be doing gigatons [of CO2] by 2025,” Nemet says. “What they need is a sequence of increasingly large facilities they learn from and improve to get the cost down over time.”
Oman is an attractive location for more than its rocks; the country’s big fossil-fuel industry has expertise handling pressurized gases, and sunlight is intense year-round. An operation capturing a billion tons of gas a year would require 700 billion to 1.3 trillion kilowatt-hours of energy, according to McQueen’s calculations. This power could come from 300 to 600 square kilometers of solar arrays, occupying no more than 0.2 percent of Oman’s territory, according to standard formulas based on sunlight intensity.
Oman is also known for its dramatic coastlines, canyons, medieval fortresses and mosques, which attract millions of tourists every year. And it is home to a traditional Bedouin population that migrates seasonally. This fragile heritage must be protected, but the country, and the Arabian Peninsula as a whole, has an abundance of empty, arid land that could host a burgeoning negative-emissions industry.
Scientists have other visions about how to use mantle rocks. Some suggest, for example, that they be mined, crushed to increase their surface area, and spread across thousands of square kilometers of desert, where they would naturally absorb CO2. Every year they could be gathered and baked to drive out the CO2, then spread again. The CO2 would have to be disposed of—likely by injection into other rock formations—or used as a raw material for plastics or synthetic fuels. Alternatively, the rock could be spread on croplands and left there, where it would absorb CO2 and potentially improve the soil quality. Either way, mining, crushing and transporting the stone could scar the landscape and consume loads of energy. In Oman, at least, Kelemen’s modest proposal to drill 5,000 injection wells may seem less extreme. Wells could be located along parts of the coastline that already host industrial operations, producing no more visual disturbance than a coastal wind farm would. Solar arrays could be placed in carefully selected parcels farther inland.
For now, Kelemen says he is glad simply to see a first step: the field test in Wadi Lawayni. His journey has been a long one, from aloof curiosity in the early 2000s to outright excitement today. He is already thinking ahead about how the physical footprint of mineral carbonation could be reduced. One evening in 2018 he led me scrambling up a canyon. In the dimming light of dusk, he stopped and pointed to a reddish pinnacle to our right. “In this mountain there are a billion tons of CO2 ” he declared. Across Oman carbonate veins account for only 1 percent of the surface rock volume, but in this little mountain, Kelemen said, “every single magnesium atom and every single calcium atom are combined with CO2 to form carbonate.”
These rocks started out with the same mantle minerals found everywhere else. But they reacted with CO2 and water longer ago, while they were still deeply buried and therefore very hot. (The water and CO2 came from a deep subduction zone nearby, where ocean sediments were being pressure-cooked as they sank into the mantle.) Based on geochemical analyses published in 2020, Kelemen thinks the rocks were as hot as 250 degrees C when they mineralized the CO2—hot enough to push reaction-driven cracking to completion—so every bit of rock could react.
Plenty of Oman’s mantle rocks are that hot today, but they are five to six kilometers below the surface. Reaching them would require more sophisticated drilling, which could make economic sense if pilot studies prove out, Hasan says.
After all, the negative-emissions industry is still in its infancy, at the same stage oil drilling was at in the mid-1800s, when it was dwarfed by a mighty whale-oil industry. The first oil wells were only a few meters deep. Companies gradually pursued bigger prizes deeper in the earth. What allowed that to happen was better drilling technology; a burgeoning global infrastructure for capturing, transporting and selling the valued product; and a growing desperation for more. These same forces might one day drive humans to delve ever deeper in search of another resource: hot rocks to solidify CO2. Oman, a country that has earned billions of dollars by selling buried hydrocarbons to the world, could make the ingenious transition to earning billions more to bury that same carbon back in the ground.



