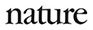Susan was still a child when she first suspected something might be wrong with her mother. A cup or plate would often crash to the floor by accident when her mother was serving dinner or washing up dishes. “She was, she would have said, ‘clumsy’, but she wasn’t really clumsy,” says Susan. “Her hands had beautiful, glamorous movements, which I now recognize as early HD.”
Huntington’s disease (HD) is an inherited condition that causes widespread deterioration in the brain and disrupts thinking, behaviour, emotion and movement. The disease usually begins in midlife, with subtle changes such as mood swings and difficulty in staying focused. As it progresses, people develop dementia and an inability to speak or move.
Susan, who requested that her last name be withheld to protect her privacy, vividly remembers the day she learnt that her mother had the disease. It was the spring of 1982, and her mother had been admitted to a hospital because of her extreme exhaustion, frequent falls and irregular movements. There was no genetic test for the condition at the time, so she underwent a series of assessments. Her neurologist gathered the entire family into a room to break the news. “He told us that our mother had Huntington’s disease,” recalls Susan. “And that there’s no treatment and it can be wiped out in a generation if you just don’t breed.”
Those blunt words had a profound impact on the lives of Susan and her siblings: her brother decided never to get married, and her sister chose to be sterilized. For Susan, however, those options were out of reach: she was pregnant when she received the news.
Susan says that she and her husband “couldn’t decide what was the right thing to do”. One thought, in particular, was that “if we have the child, then that child will have this same decision when they grow up”, she says. “And it seemed so cruel.” Ultimately, the couple made the heart-wrenching choice to terminate the pregnancy.
The gene involved in Huntington’s, called HTT, codes for a protein called huntingtin. The faulty version of the gene repeats a short piece of its sequence—the nucleotide combination CAG—too many times. Unlike some genetic conditions, in which a person will develop a disease only if they have two faulty copies of a gene, just one copy of the HTT mutation is enough to lead to Huntington’s, and carriers of the mutation have a 50% chance of passing it on to their children. Years after Susan’s mother passed away, the three siblings discovered that they had all inherited the disease.
There are no treatments available to stop or slow the progression of Huntington’s, even though its genetic cause has been clear since 1993. Most other neurodegenerative diseases also lack effective therapies and, although their genetic roots are less clear-cut than for Huntington’s, many of the genes associated with conditions such as motor neuron disease (amyotrophic lateral sclerosis, or ALS), Alzheimer’s and Parkinson’s have been known for decades. Now, the tide might be turning for treating these kinds of diseases. Many researchers are hopeful about drugs known as antisense oligonucleotides (ASOs). These are short strings of DNA or RNA letters that are designed to cling to particular sequences of RNA made by faulty genes, and to rebalance the levels of proteins they produce—boosting missing proteins or quashing faulty ones (see ‘Toggling problem proteins’).
The US Food and Drug Administration (FDA) approved the first ASO for a neurological disease in 2016, and there has since been an explosion of activity in this area. The field has gone from just a handful of clinical trials run over the past two decades to around a dozen currently under way for various neurodegenerative diseases—and a few have reached their final stages.
Other ASO researchers are moving beyond diseases defined by a single mutation to look at conditions with more-complex genetic underpinnings. This recent progress has made many in the area optimistic about the future of the technology. Don Cleveland, a neuroscientist at the University of California, San Diego (UCSD), and one of the first scientists to investigate the use of ASOs for neurological diseases, sees this as just the beginning. “There’s much, much more coming,” he says.
But progress in the field has not been completely smooth. At the end of last month, a large phase III trial was abruptly halted because the benefit of the drug to patients did not outweigh the risks. And some researchers have long urged caution around ASOs, owing to the fact that their efficacy in many conditions is unknown and that the way they are delivered—often by spinal injection—is invasive.
Although the outcome of this trial was disappointing, “I don’t think this is a reason for despair,” says Chris Boshoff, a scientific-project manager overseeing genetic therapies at the US National Institute of Neurological Disorders and Stroke in Bethesda, Maryland. “There’s still reason to be positive and enthusiastic about what this modality can accomplish.”
Breakthrough for a rare disease
Elliot and Janell Lewis’s first child, Blakely, was born in 2011 with a rare, inherited neurodegenerative disease known as spinal muscle atrophy (SMA). People with SMA have a mutated form of SMN1, a gene responsible for producing a protein called survival motor neuron (SMN). The resulting lack of SMN prevents the brain from being able to communicate effectively with the body, leading to muscle weakness and wasting that worsens over time. There are four types of SMA; the most common form, SMA1, is also the most severe. People with SMA1 typically show symptoms shortly after birth, and many do not survive past the age of two.
Blakely was diagnosed at three months old. “That pretty much shattered us,” Elliot says. At the time, there was no treatment, and Blakely passed away at 21 months.
In the spring of 2017, the couple had another daughter, Evie. Evie also had SMA, but she was more fortunate—a few months before she was born, the FDA approved an ASO, dubbed nusinersen, the first ever disease-modifying treatment for SMA. Evie received her first dose when she was 12 days old.
 SMA1035.jpg)
Scientists first recognized the ability of ASOs to target RNA in 1978, but it took several decades to demonstrate their clinical potential. Early on, problems such as toxicity and lack of potency stymied progress, and many drug companies lost interest. But researchers at one firm, Ionis Pharmaceuticals (originally named Isis Pharmaceuticals), based in Carlsbad, California, introduced key modifications to the drugs’ chemical backbone that increased potency as well as stability, enabling the ASOs to reach their targets without being degraded.
The work that led to nusinersen began around 2000 at Cold Spring Harbor Laboratory in New York, where biochemist and molecular geneticist Adrian Krainer was investigating the mechanisms that led SMN2, another gene that encodes SMN, to typically produce less viable protein than its counterpart, SMN1. They reasoned that if they could get SMN2 to produce more protein, it could make up for SMN1 in people with a mutation in that gene. They knew from others’ work that in almost everybody, the cause of the problem with SMN2 was an error during splicing—the process through which strands of RNA are snipped and processed into instructions for making proteins. That causes a piece of SMN2’s code to be skipped.
Krainer’s team zoomed in on the proteins that bind to the RNA strand and cause the segment to be missed, hoping to stop them interfering in the process of generating complete SMN proteins. In 2004, Krainer began collaborating with Frank Bennett, a pharmacologist and one of the founding members of Ionis Pharmaceuticals. Together, they pinpointed an ASO that could bind to the strand and hide the segment from the proteins that would silence it, enabling the production of functional SMN.
That compound, nusinersen, entered clinical trials in 2011. The results were so promising that the phase III trial in infants with SMA was terminated early: patients who received the drug were much more likely to meet their motor milestones and survive than were those who received a placebo.
So far, more than 10,000 people worldwide have received nusinersen (Spinraza), which Ionis licensed to drug maker Biogen, based in Cambridge, Massachusetts, in 2016. The drug has drastically altered the course of the disease: infants with SMA who receive it shortly after birth are no longer dying within the first years of life. Nowadays, “conversations [with families] don’t just end with, ‘We’re going to do everything we can, but your baby’s going to die’,” says Russell Butterfield, a paediatric neurologist at the University of Utah in Salt Lake City (Butterfield has received consulting payments from Biogen). “Instead, that conversation switches to, ‘We have this new drug, it’s absolutely amazing. We need to get it in as soon as possible’.”
Evie Lewis, now four years old, receives a dose of Spinraza by a lumbar puncture every few months, and she recently had her 15th injection. Although she still faces some issues, such as having to eat through a feeding tube, she is able to walk, run and climb—things that Blakely was never able to do, Elliot says.
A packed pipeline
Following the success of nusinersen, researchers began to tackle other diseases associated with clearly defined genetic mutations, such as Huntington’s. That led to the drug tominersen, which was developed by Ionis and licensed for clinical testing to the pharma company Roche in Basel, Switzerland. It is thought to work by targeting CAG repeats on the RNA strand produced by both the normal and faulty HTT genes, and tagging them for destruction by an enzyme called RNase H1. The results of a phase I/II clinical trial, which were published in 2019, revealed that tominersen lowered concentrations of the mutant version of huntingtin in the cerebrospinal fluid, without causing any serious side effects.
The success of the early Huntington’s trial caught the attention of neurodegeneration researchers, because tangles of protein are a key feature of many such disorders. “There was a lot of excitement about this, because it really opened up the doors to be able to do antisense trials for other neurodegenerative diseases where build-up of a toxic mutant protein plays a role,” says Sarah Tabrizi, a neurologist at University College London, who led the phase I/II trial of tominersen.
But an unexpected announcement at the end of March dealt a big blow to the Huntington’s community. A phase III trial of tominersen involving 791 participants from 18 countries was terminated early on the advice of an independent committee of experts, who had conducted a planned review of the data. A statement from Roche said that no new safety concerns had emerged, but that the drug’s potential benefits did not outweigh the risks. Until more details are published, it’s not possible to say what went wrong, says Tabrizi.
Drugs that work in a similar way to tominersen are still in play for other disorders with similar causes. Some cases of ALS, for instance, are caused by too much of a mutant protein, and a handful of ASOs for those forms of the disease are in clinical trials. The furthest along is tofersen, an ASO developed by Ionis to treat an inherited form of ALS. Tofersen is now being tested in a Biogen-sponsored phase III trial.
Claudia Testa, a neurologist at Virginia Commonwealth University in Richmond, says that there are unique challenges that come with reducing the levels of a mutant protein, as tominersen and tofersen do, compared with boosting a missing one, as nusinersen does. Several protein-lowering strategies actually reduce levels of both good and bad versions of a protein. Scientists do not yet know the long-term effects on the diseases concerned, and it’s not clear if this was the issue in the phase III trial of tominersen. The drug for SMA is doing something fundamentally different, “so it doesn’t predict efficacy for the other diseases—and that’s a painful truth”, says Testa.
To avoid this problem, some ASOs are aimed squarely at mutant proteins. One biotechnology company, Wave Life Sciences in Cambridge, Massachusetts, is testing a strategy that targets tiny mutations that sometimes occur alongside the CAG repeats on just the mutant copy of HTT. The aim is to leave levels of healthy huntingtin relatively intact. But the drug would work only in a subset of people with Huntington’s who carry these mutations. Furthermore, that difference can be identified only with an exhaustive sequencing method that is not routinely carried out in the clinic, says Testa. (Testa has received consulting fees from Wave Life Sciences.)
More recently, researchers have started testing ASO-based therapies for more-common neurodegenerative conditions, such as Parkinson’s and Alzheimer’s. The vast majority of cases are not linked to a specific genetic mutation, and these disorders are much more prevalent than are inherited diseases. The ASO for Alzheimer’s aims to lower levels of tau, a protein that builds up into toxic tangles in the brain. For Parkinson’s, the goal is to lower the α-synuclein protein, which aggregates into pathological clumps known as Lewy bodies.
But for neurogenerative diseases such as these, several genes in a network are likely to be involved, says Kevin Talbot, a neurologist at the University of Oxford, UK, who will be involved in a forthcoming trial of an ASO for ALS. It’s unclear how a change to one gene in the network would affect the rest, he says. (Talbot has previously served on scientific advisory boards for Roche and Biogen.)
Another issue, according to Talbot, is that these drugs currently need to be delivered using repeated lumbar punctures to reach their targets in the central nervous system. Before ASOs can be applied to a wider range of diseases, it will be important to find a way to get these drugs past the blood–brain barrier so as to deliver them less invasively, Talbot says. “There’s a whole list of things that have to be done before we get too triumphalist.”
Change of identity
Studies in mice suggest that the ASOs of the future could have even more powerful uses in the brain: replacing lost neurons.
Last year, Xiang-Dong Fu, a cell biologist at UCSD, and his colleagues demonstrated that it is possible to use ASOs to convert non-neuronal brain cells called astrocytes into neurons. The team injected an ASO into a region of the mouse brain from which neurons are lost in Parkinson’s. Once there, the drug activated a network of genes that prompts astrocytes to become neurons. In mouse models of Parkinson’s disease, Fu’s team found that animals that received the treatment showed improvement in certain behaviours.
Cleveland, who was involved in Fu’s trial, has been working with an ASO supplied by Ionis to test the idea in other parts of the brain. “This is really where I’m going to invest the rest of what I’ve got left as a career,” he says. “I’m confident that we have only begun to think about the possibilities.”
These astrocyte-converting ASOs are still at an early stage. Fu cautions that before this technique is taken to the clinic, it needs to be tested in non-human primates, because their brains match our own more closely than do those of mice.
For now, researchers are eagerly awaiting the results of the tofersen phase III trials in ALS, and for more information about exactly why the tominersen trial for Huntington’s was halted.
Susan, a retired nurse in her mid-60s, has been involved in the tominersen trial since phase I. She is disappointed in the news, she says, but is grateful for the care she’s received as a participant. “I’ve been so privileged to be part of this trial right since day one. Now it’s just about patience and reviewing. There’s no alternative, is there?”
This article is reproduced with permission and was first published on April 6 2021.