Over the centuries treatment of mental illness has shifted dramatically. At present, drugs that manipulate neurochemistry dominate therapeutics. In earlier times, the heights of supposed efficacy and compassion were lobotomies and insulin-induced comas. Earlier still, restraints and ice baths sufficed. Go further back, and we enter the realm of exorcisms.
Despite this progress, better treatments are desperately needed, particularly for anxiety and depression. The former, the most common mental illness in the U.S., afflicts 15 to 20 percent of adults in America, and the latter is among the leading causes of medical disability worldwide. Moreover, they were this widespread before 2020; in the COVID-19 era, anxiety and depression have become pandemics of their own.
In this article, I review the brain bases of anxiety and depression (although both words describe everyday transient states that can trouble all of us, throughout I am referring to the medical diagnoses of anxiety disorders and major depression, diseases that incapacitate their sufferers chronically). The study of these ailments readily generates dichotomies: Are anxiety disorders and major depression fundamentally biological or psychological in nature? Do they arise mostly from nature or nurture? But these are false dichotomies, and I will consider here how stress creates links between the neurobiological and the psychological and between genetic and environmental factors. As we will see, although the two classes of diseases differ in their presentations, the role of stress as a risk factor for both reveals their deep similarities.
Biology of Anxiety
Anxiety comes in various subtypes, such as generalized anxiety disorder, social anxiety disorder, specific phobias and panic attacks. Despite this heterogeneity, they all involve the serious disruption of normal function by a consistent tendency to see particular circumstances as threats that most other people do not perceive. Given that, it is logical that the brain region whose abnormal function is most implicated in anxiety is the amygdala. This structure is a core part of the limbic system of the brain, a region central to emotions that, while responsive to an array of negative stimuli, is most involved in the perception of and response to fear-evoking stimuli.
Its actions can be highly adaptive. If a lion is stalking you, the activation of the amygdala mediates your appropriate responses to the fearsome circumstance. If your amygdala reacts with the memory of another time that a lion stalked you, its activation helps to consolidate information about what you did to escape. If your amygdala reacts as you approach an African streambed that might harbor a lion, its activation prepares you in case the fear is justified. But if you believe that there will always be a lion waiting for you when you exit an elevator, the activation of the amygdala encapsulates the maladaptive nature of anxiety. This, in turn, can produce a life filled with the corrosive perception of threats to health, safety and well-being that are simply not there.
Unsurprisingly, a consistent finding is that anxiety disorders are associated with chronic hyperactivation of the amygdala and of the brain regions it in turn stimulates—regions involved in the activation of the sympathetic nervous system and its release of epinephrine (also known as adrenaline), along with the secretion of other stress hormones, all preparing the body for fight or flight.
For the amygdala to deal with an ongoing threat, it must receive information about the surrounding world. Such sensory information is first processed in the cortex, which decodes and interprets it, supplying meaning, context and associations before notifying the amygdala. Such integration allows categorization so that, for example, pictures of hands being washed, masked people spaced six feet apart, and a respirator now all trigger the same kind of anxiety in people who endured the stress of 2020.
Crucially, the amygdala also receives sensory information that bypasses the cortex by way of a shortcut. As a result, a potential threat can activate the amygdala before there is conscious awareness of it. This rapid transmission comes with a price: accuracy is sacrificed for speed. This is the world where, with just an instant to react, a cell phone is mistaken for a handgun, and a trigger is pulled. As shown both experimentally and in real life, the likelihood of that mistake varies depending on the race, age and gender of the cell-phone holder, placing the amygdala at the center of an all too familiar relation between bias and tragedy.
The amygdala also receives input from the insula, a region in the brain that processes sensations about the body’s internal state (known as interoceptive information)—a stomach rumbling, an achy elbow, a dry mouth before speaking in public. Listening to your heart with a stethoscope unsettles most of us—hearing the beats reminds us of mortality. As shown with neuroimaging, the experience activates the insula, which then activates the amygdala. People with anxiety disorders are especially vulnerable to this phenomenon; greater degrees of insula activation predict greater subjective anxiety. Perhaps this is the neurobiological explanation for those for whom every random twinge of bodily pain suggests a fatal disease.
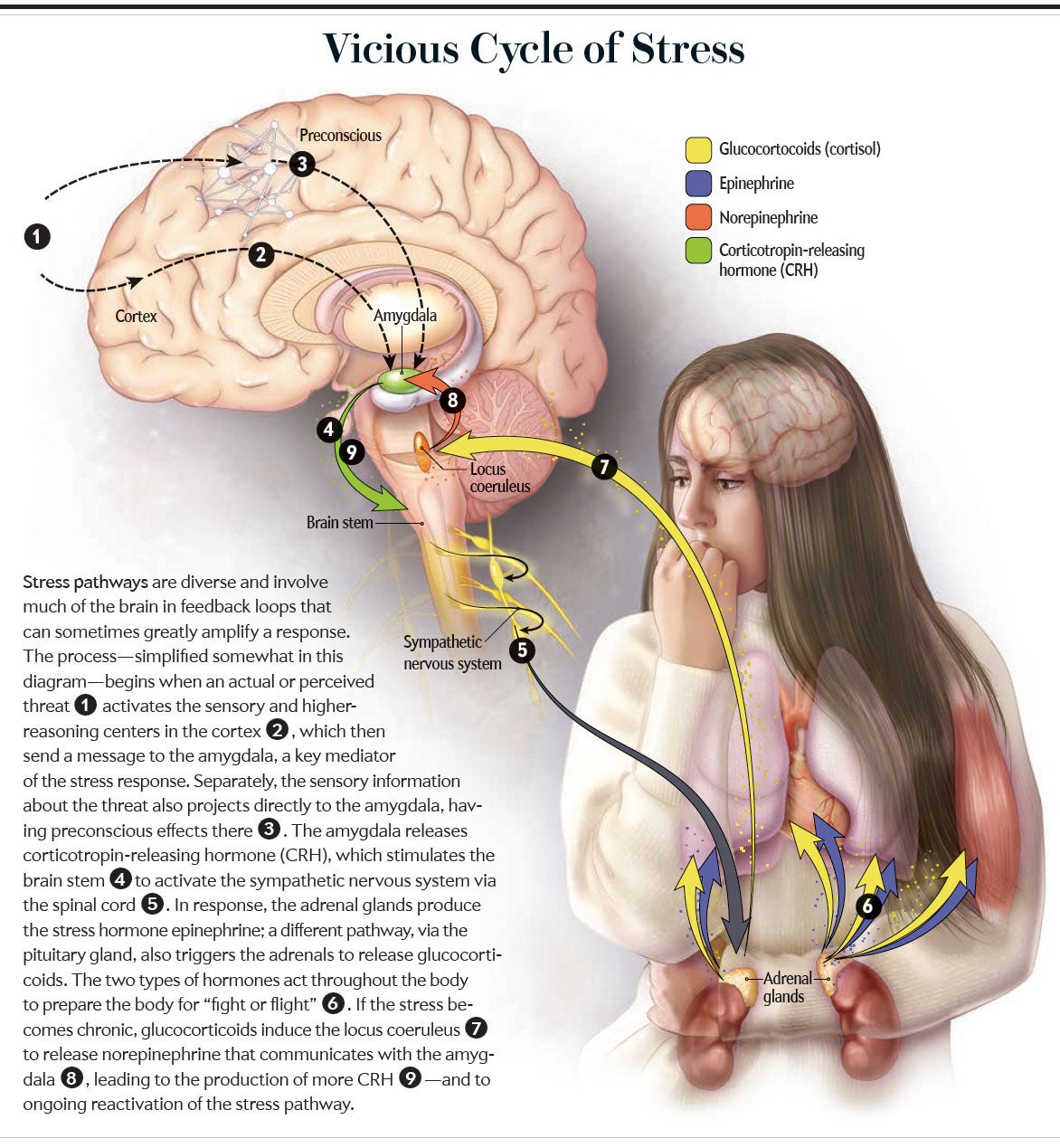
The amygdala also receives input from additional brain regions. One, the locus coeruleus, uses the neurotransmitter norepinephrine, sometimes called noradrenaline; its activation triggers arousal, alertness and vigilance throughout the brain—particularly in the amygdala. Those with anxiety disorders who experience a typical increase in locus coeruleus activity have exaggerated startle responses.
The amygdala also receives projections from the prefrontal cortex (PFC), a brain region central to executive function, emotional regulation and impulse control. Suppose you were conditioned yesterday to associate a bell with a shock such that by the end of the day, the sound of the bell alone activated your amygdala. But today things have changed: the bell is no longer followed by a shock. At first the ring of the bell activates the amygdala as before. But as the ringing repeats without shocks, the PFC activates, gradually inhibiting the amygdala. Thus, the input from the PFC to the amygdala mediates our ability to discern that we are now safe, extinguishing the amygdala’s responsiveness. Not surprisingly, this input is fainter in anxiety disorders; as we will see, a weakened PFC has even more implications.
After this overview, one can appreciate some of the drugs used most often to treat anxiety. Most enhance the potency of the inhibitory neurotransmitter GABA (gamma-aminobutyric acid). It produces a sedating effect, and the drugs used to accomplish this (benzodiazepines such as Valium) are known as minor tranquilizers. Another strategy that has shown some success experimentally, including for the treatment of post-traumatic stress disorder, involves preventing effects of norepinephrine on the locus coeruleus by blocking its receptor with beta-blocker drugs such as propranolol. Some people self-medicate their anxiety disorders by using cannabis, which produces anti-anxiety effects by binding to cannabinoid receptors in the brain, including in the amygdala.
With this brief overview, we now turn to the basic biology of depression, which will prepare us to appreciate the role of stress in the emergence of both disorders.
Biology of Depression Basics
As with anxiety, major depressive disorders come in subtypes, but they all involve a persistent and disabling excess of negative emotions in conjunction with a shortage of positive ones. This imbalance underpins the defining symptom of depression, namely, anhedonia, the inability to feel pleasure. In depression, however, even more fundamental than the inability to feel pleasure is the inability to anticipate it or to feel the motivation to pursue it. Add to that the symptom of rumination, which in this context means sad thoughts cannot be stopped; the mind churns from one to the next, with interspersed feelings of helplessness and self-hatred. From this pattern comes the classic, poetic characterization of depression as “aggression turned inward” and the tragic suicidality common to the disorder. Concentration, appetite and sleep are typically impaired, and the person becomes mired in “psychomotor retardation,” making it exhausting to function in the most everyday ways. High blood levels of glucocorticoids (cortisol in humans), stress hormones secreted by the adrenal glands, are typically found with major depression.
Because anhedonia is at the core of major depression, the neurotransmitter dopamine plays a central part. Although it serves various functions in the brain, the most pertinent is its role in the ventral tegmentum, a region that sends dopamine-releasing projections throughout the limbic system and the frontal cortex. This circuitry was originally termed the dopaminergic reward system, a bit of a misnomer in that the neurotransmitter is even more important for detecting salient cues about the possibility of reward, the anticipation of it, and the goal-directed, motivated behaviors needed to attain it. As one might predict, in cases of major depression, this dopaminergic system is less responsive than normal to positive stimuli and cues about reward.
As reviewed, although the amygdala responds to stimuli related to threats, it also responds to other negative stimuli. In major depression, the amygdala is overreactive to various such stimuli, especially those evoking sadness. Although this could be viewed as an amygdaloid function unrelated to fear, one also could reframe this finding in terms of threat. After all, for someone with major depression, few things are as menacing as a stimulus prompting yet more sadness.
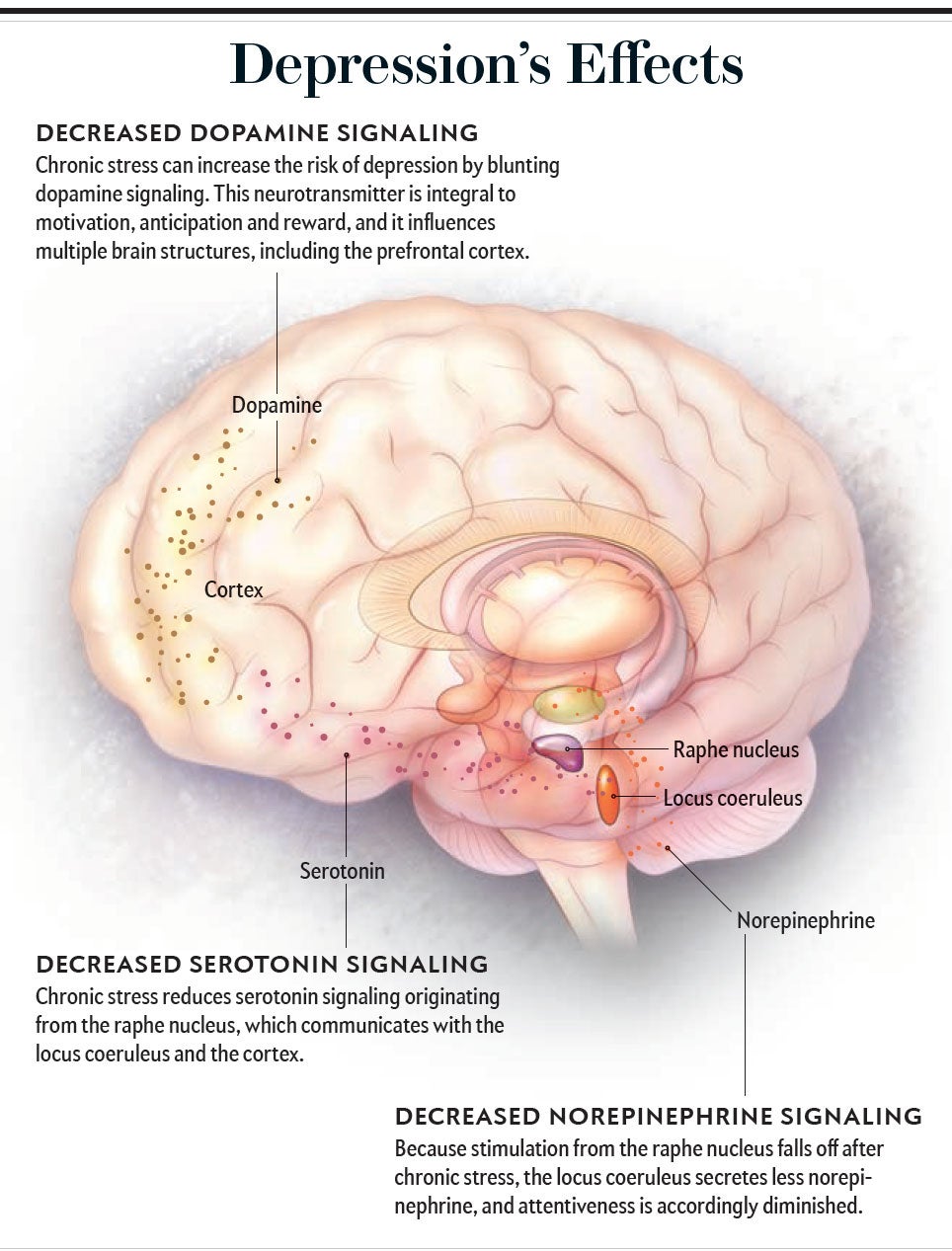
Long-term major depression also causes changes in the hippocampus, a brain structure central to learning and memory, namely, atrophy, along with impaired hippocampal-dependent cognition. Pioneering work by the late Ronald S. Duman of Yale University tied these changes to a brain growth factor, brain-derived neurotrophic factor (BDNF), showing in both laboratory animals and humans that depressive states are associated with BDNF shortages in the hippocampus. Moreover, successful treatment of depression with medications reverses the paucity of BDNF, and the efficacy of such drugs might even require this effect on BDNF. Finally, major depression is associated with atrophy and weakened function of the PFC, with, again, depletion of BDNF implicated in the phenomenon (we will revisit the consequences of PFC dysfunction in depression further on).
Along with the underactivity of the dopaminergic reward system, there also appear to be decreases in signaling mediated by norepinephrine and the neurotransmitter serotonin. These changes are the basis for the standard pharmacological strategy for treating depression, that is, using drugs that boost signaling in all three neurotransmitter systems. All block the removal of neurotransmitters from the synapse, allowing them to persist and act longer. The classic examples are the selective serotonin-reuptake inhibitors (SSRIs—drugs such as fluoxetine, sold under the brand name Prozac); dopamine and norepinephrine reuptake also can be enhanced by other drugs such as bupropion. The neurotransmitter glutamate has been implicated in depression more recently: ketamine, a drug that potently blocks a subtype of glutamate receptor, can rapidly lessen severe, intractable depression. Why this works remains mostly unknown; nevertheless, the 2019 approval of ketamine as a treatment for persistent depression by the U.S. Food and Drug Administration represents the first introduction of a new class of antidepressant medications in decades.
Being Out of Balance
So how does stress impact both anxiety and depression?
When a body is in homeostatic balance, measures such as temperature, glucose level, and so on are in the “ideal” range. A stressor is anything in the environment that disrupts homeostasis, and the stress response is the array of physiological adaptations that reestablish balance. Many of these adaptations arise from activation of the sympathetic nervous system and secretion of glucocorticoids.
For most mammals, stressors are acute physical challenges such as evading a predator or pursuing a meal. Epinephrine and glucocorticoids mobilize energy for muscles, increase cardiovascular tone to deliver oxygen and glucose rapidly to those muscles, and inhibit unessential processes such as growth, digestion and reproduction until more auspicious times.
For us primates and other socially sophisticated species, the same stress response can be activated by subtler things than a predator, including thoughts and feelings about other members of our species. This concept brings us to the realm of psychosocial stress. Starting in the 1950s pioneers such as John Mason, Seymour Levine and Jay M. Weiss—then at Walter Reed Army Medical Center, Stanford University and the Rockefeller University, respectively—began identifying key facets of such stress. For the same external stressor, there is a greater subjective sense of stress, a stronger stress response and a higher risk of stress-related disease if there is no outlet for frustration, no sense of control or predictability, no social support and no optimism. Thus, a rat will be less likely to develop an ulcer after a series of mild shocks if it can gnaw on a bar of wood throughout the stressful period as an outlet for frustration. A baboon will secrete fewer glucocorticoids in response to frequent fighting if the aggression results in a rise, rather than a fall, in that baboon’s rank in the dominance hierarchy; he will perceive life as improving. A person will become less hypertensive when exposed to painfully loud noise if there is a warning signal five seconds before each blast because she will be receiving predictive information.
But suppose such buffers are not available and the stress is chronic. Repeated challenges may demand repeated bursts of vigilance. At some point this vigilance may become overgeneralized, leading an individual to conclude that she must always be on guard even in the absence of the stress. This person will have entered the realm of anxiety. Alternatively, these insurmountable stressors can produce feelings of helplessness that may become overgeneralized, leading a person to conclude that he is always helpless even when that is not the case. Depression is then upon him.
Thus, we see the links between chronic stress as a psychological phenomenon and the symptomatology of anxiety and depression. This picture fits well with the neurobiological effects of stress.
Sustained stress, by way of excessive glucocorticoid secretion, makes synapses in the amygdala more excitable; furthermore, amygdala neurons grow new connections, even to the point of increasing the amygdala’s volume. Moreover, the sensory shortcut to the amygdala that bypasses the cortex is strengthened. Meanwhile glucocorticoids will be having opposite effects in the PFC—reducing synaptic excitability, atrophying neuronal connections, and suppressing the synthesis of BDNF, which would otherwise rebuild those connections. These stress effects produce an amygdala that learns fearful associations more readily and a PFC that is less capable of extinguishing them—our precise neurobiological depiction of anxiety.
As noted, depression is characterized by hyperreactivity of the amygdala (albeit in ways that differ from anxiety) and weakening of the projections to it from the PFC; again, chronic stress and an excess of glucocorticoids bring these changes about. In addition, sustained stress can deplete the locus coeruleus of norepinephrine, with one result being the psychomotor retardation of depression. Most important, chronic stress also impairs the dopaminergic reward system, with evidence for both depletion of dopamine and decreased sensitivity of neurons to it. We now have the neurobiological roots of anhedonia, the loss of anticipation of and the motivation to pursue pleasure.
Transition from Anxiety to Depression
Thus, chronic stress can produce the neurobiological profiles of both anxiety and depression. But the conviction that threats are everywhere and a feeling of ceaseless sadness are very different emotional states. As we have seen, neurobiologically, anxiety and depression are different malaises. Nevertheless, new insights show their similarities, particularly with respect to the role of stress.
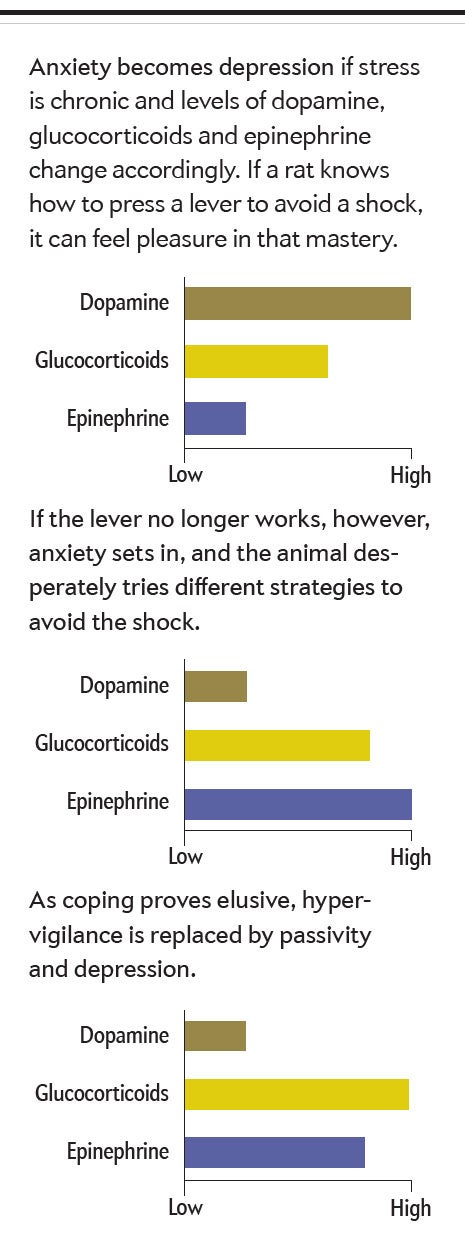
One important commonality is how anxiety can segue into depression. Consider a rat trained to press a lever to avoid mild shocks—a task readily mastered. The anticipatory sense of mastery might well activate the rat’s dopaminergic projections to the frontal cortex. And when glucocorticoid secretion is moderate—as would be the case in this scenario—the hormone enhances dopamine release.
Suppose, however, that the lever has been disconnected; pressing it no longer prevents shocks. Initially wildly reactive hypervigilance occurs in the rat as it presses the lever continually or begins other repetitive behaviors, hoping to regain control over the shocks. When these disorganized, frantic behaviors persist long after the shocks have stopped, along with chronic sympathetic activation and elevated epinephrine secretion, we see the essence of anxiety.
But in some rats, as the shocks continue and coping attempts fail, a transition takes place: the coping behaviors stop—the animal has learned to be helpless. The stress response becomes more dominated by high glucocorticoid levels than by epinephrine, and brain chemistry begins to resemble that seen in depression. If anxiety is a crackling brushfire, depression can be a suffocating quilt thrown on top of it.
In other words, anxiety and depression can coexist, with one dominating the other in a context-dependent way. For example, anxiety might come to the fore when one is contemplating a roomful of strangers, whereas depression dominates when that person contemplates global warming. Unsurprisingly, a substantial percentage (perhaps more than 50 percent) of individuals with either disease suffer from both, compounding the therapeutic challenges.
Anxiety and Depression as Cognitive Disorders
As underscored by their designation as mood disorders, anxiety and depression are diseases of abnormal emotion. But they are also diseases of abnormal cognition. As pioneered by the renowned psychiatrist Aaron T. Beck (who is celebrating his 100th birthday in 2021), cognitive-behavioral therapy (CBT) focuses on that component of the diseases. CBT aims to uncover the cognitive distortions that lead from a truism of “that happened to me” to the erroneous worldview of “that will always happen to me” and to give the sufferer the cognitive strategies to counter such distortions. CBT is among the most successful psychotherapeutic approaches to the treatment of both diseases, showing the strength of their cognitive components.
The diseases share an additional, related cognitive component. As reviewed, both involve features of a weakened PFC, with decreased metabolism, particularly in the face of enhanced amygdaloid activity, and uncoupling of the ability of the PFC to regulate the amygdala. Depression seems to cause actual cell loss (predominantly of glial cells rather than neurons); anxiety atrophies PFC projections to the amygdala (as shown by decreased density of white matter, the insulation around neuronal cables). These various changes are produced by both hyperactivity of the locus coeruleus and glucocorticoid excess.
What are the consequences of a weakened PFC? One classic series of studies (with healthy volunteers) showed the normal functioning of the PFC in this regard. In these experiments, a subject in a brain scanner plays a virtual game of catch on a computer connected to two other “participants” (in actuality, the other two players are part of a computer program). A cyber ball is tossed back and forth among the test subjects; suddenly, for no obvious reason, the other two supposed players snub the human subject and begin tossing the ball just between themselves. In most individuals, this causes a burst of activity in the amygdala, a torrent of negative affect tied to the social insecurities lurking inside us. Then, after a short delay, the PFC typically activates and asserts cognitive control by reappraising the situation (“It’s just a game; outside this stupid scanner I have friends and loved ones”) and quiets the amygdala.
This is the role of the PFC that is impaired in both disorders. Anxiety and depression can both be thought of as disorders where the PFC is unable to exert cognitive control over adverse emotions. This failure to stop negative thoughts in their tracks leaves the sufferer mired in vicious perseveration on the negative and seems to involve serotonin shortages in both diseases. As evidence, drugs that boost serotonin signaling (such as the SSRIs) ease both anxious and depressive ruminations (as well as the intrusive thoughts and repetitive rituals of obsessive-compulsive disorder).
An insightful definition of depression is that it is the pathological failure of rationalization, and the same applies to anxiety. Whether the wall we put up against the flooding sea is built on facts or on the self-deceptions that often sustain us, its failure in anxiety and depression shows that these are diseases not only of abnormal emotion but also of abnormal regulation of emotion.
Childhood Stress and Its Epigenetic Legacy
When faced with chronic stress, only some individuals fall into anxiety or depression. Differing extents of early life stress help explain the differing thresholds for stress-induced mood disorders.
A childhood filled with adversity, trauma or loss can obviously leave scars for a lifetime, including psychiatric ones. Developmental psychologists now formalize such histories with ACE scores (for “adverse childhood experiences”), a tally of a person’s childhood experience of physical, emotional or sexual abuse; physical or emotional neglect; and unstable home life because of a family member being incarcerated, addicted, violent or mentally ill. These assessments have revealed a dishearteningly clear linear relation: the higher a child’s ACE score, the greater the likelihood of anxiety or depression in adulthood (along with other heartbreaking outcomes related to educational attainment, antisocial behavior, risky sexual behavior, socioeconomic status and health).
This is no surprise. Childhood is when we first learn answers to two fundamental questions: whether the world is benevolent or malevolent, and whether you can control how your life is turning out. The neurobiological consequences of the answers pertain to at least two domains.
The first concerns how the stressors that make for high ACE scores shape brain development. Sustained childhood stress can impair maturation of the frontal cortex and the hippocampus, prompt the excessive amygdala development that leaves one destined to see threats that are not there, and even decrease the number of dopamine neurons formed. Many of these effects are mediated by excessive glucocorticoid secretion during a stressful childhood.
These effects of stress can also occur during fetal development. Excessive levels of glucocorticoids in the mother’s circulation caused by significant maternal stress will elevate glucocorticoid levels in the fetal circulation. When they permeate the fetal brain, these glucocorticoids have damaging effects that are similar to those of glucocorticoid excess in childhood. Environmental effects most certainly do not begin at birth.
Thus, extreme stress beginning in utero and extending into childhood can produce a brain destined to be more vulnerable to anxiety and depression in adulthood. But such childhood adversity alters not only what is, in effect, the hardware of the adult brain but also the software—the very genetic regulation of the adult brain, whose function was established during childhood.
Experience does not change our genes (that is, the DNA sequence that determines the precise protein encoded by each gene). Experience does, however, cause long-lasting or even lifelong changes in the regulation of genes. Such “epigenetic” effects can determine how readily a particular gene in a particular part of the body and in a particular context is activated, thereby determining how much of its cognate protein is made. As first shown by Michael J. Meaney of McGill University and expanded on by other scientists, early-life stress causes epigenetic changes in this article’s usual suspects: genes related to serotonin, dopamine and norepinephrine; to brain sensitivity; to glucocorticoids; and to growth factors such as BDNF. And these are changes that uniformly increase adults’ vulnerability to anxiety and depression.
Genetics and the Experience of Stress
Despite the crucial impact of early-life stress on adult mental health, many, perhaps even most, people who experience extreme childhood adversity do not develop into adults who suffer from mood disorders. What explains such individual differences?
The answer may lie in genetics. As noted, the DNA sequence that composes a particular gene specifies the precise construction (and thus function) of a particular protein. Crucially, most genes are polymorphic—that is, they come in multiple forms, with small differences in the DNA coding sequences changing the efficacy of the protein produced. This is the rationale for hugely ambitious cutting-edge research into variability across the entire genome in hundreds of thousands of people that aims to identify gene variants that are statistically associated with particular traits. Such studies, known as genome-wide association studies, have produced results of daunting complexity–for example, variants of hundreds of genes contribute to a trait as simple as height. Small surprise, then, that genetic surveys have implicated enormous numbers of genes in mood disorders, with none popping out with disproportionate predictive power. Nevertheless, many of the genes implicated are related to now familiar factors: neurotransmitter systems, glucocorticoid signaling and BDNF.
One might conclude, then, that a particular array of gene variants destines someone to inevitably develop a mood disorder. This picture of genetic determinism is wrong, however. Instead there is genetic vulnerability in which the interaction between such genetic profiles and stress, especially in childhood, synergistically increases disease risk.
The classic example was identified in 2003 by Avshalom Caspi of Duke University and his colleagues. They focused on the gene SLC6A4, which encodes the serotonin transporter, a protein that removes serotonin from the synapse to terminate its actions. It is a polymorphic gene, and lab studies predicted that one variant would increase the risk of depression. Caspi and his team showed that possessing that variant did indeed have that consequence—but only when coupled with major childhood stress. As further support for this finding, different variants of the gene are regulated differently by glucocorticoids. This variation constitutes a textbook example of a gene-environment interaction, in which the effect of a particular gene variant depends on the environment, and the effect of a particular environment depends on an individual’s genetic makeup.
Despite hundreds of studies concerning this gene-environment interaction, the basic finding about the serotonin transporter is immersed in controversy because it has been less consistently replicated in the most thorough studies with the largest sample sizes. Despite this conflict, it has been a model for an array of subsequent genetic results (personally, I retain faith in the veracity and importance of the original finding). Crucially, variants in a number of our usual-suspect genes predispose us to anxiety or depression, but only when coupled with early-life stress. These are critically important observations.
Into the Future
Taken together, anxiety disorders and depression are diseases of excessive negative affect arising from stress coupled with impaired cognitive control over those negative emotions. An adult’s vulnerability to either disorder is increased by early-life stress and its epigenetic consequences. In turn, the impact of early-life adversity is modulated by variability in genes related to vulnerability.
Even with this synthesis, our understanding of the role of stress in anxiety and depression is woefully incomplete. Two issues stand out to me. One concerns the fork in the road separating the two diseases. Not only do anxiety and depression share a framework of genetic vulnerability interacting with early-life stress, but many of the genes and neurotransmitter systems implicated in these diseases (as well as the drugs used to treat them) are the same for both. How can such similar biology give rise to such different outcomes?
There is another domain where more insights are urgently needed. Despite the often bitter exigencies of life, most of us do not succumb to anxiety or depressive disorders, and there are people who are spared despite the most nightmarishly anxiogenic or depressogenic circumstances. It is critical to understand the psychobiology of resilience, which must consist of far more than just the absence of risk factors; this is an area of active research.
Two last take-home messages also come to mind. First, our exploration of the genetics, epigenetics, endocrinology and neurobiology of these diseases underlines the fact that they are diseases, as real as, say, diabetes or cancer. This should be recalled by those spared these mood disorders if they are tempted to view a sufferer as self-indulgent or lacking the backbone to just overcome their problems like everyone else does. Finally, it should be self-evident that something is wrong when a society is so unequal as to teach some of its members that life consists of menace and that they are fundamentally helpless; the incidences of anxiety and depression soar among the lower rungs of the socioeconomic ladder. Neuroimaging or genome-wide association studies should not be necessary to make that point palpable. But insofar as they seem to be, findings such as those discussed here should be known far and wide.

