The United States leads the world in COVID-19 deaths but lags behind many countries—both large and small—in testing capacity. That could soon change.
At the end of August, the US Food and Drug Administration (FDA) granted emergency-use approval to a new credit-card-sized testing device for the coronavirus that costs US$5, gives results in 15 minutes and doesn’t require a laboratory or a machine for processing. The United States is spending $760 million on 150 million of these tests from health-care company Abbott Laboratories, headquartered in Abbott Park, Illinois, which plans to ramp up production to 50 million per month in October.
The tests detect specific proteins—known as antigens—on the surface of the virus, and can identify people who are at the peak of infection, when virus levels in the body are likely to be high. Proponents argue that this could be a game changer. Antigen tests could help to keep the pandemic at bay, because they can be rolled out in vast numbers and can spot those who are at greatest risk of spreading the disease. These tests are also a key element in the testing strategies of other countries, such as India and Italy.
Antigen assays are much faster and cheaper than the gold-standard tests that detect viral RNA using a technique called the polymerase chain reaction (PCR). But antigen tests aren’t as sensitive as the PCR versions, which can pick up minuscule amounts of the SARS-CoV-2 virus that causes COVID-19.
This difference raises some concerns among specialists, who worry that antigen tests will miss infectious people and result in outbreaks in countries that have largely controlled coronavirus transmission. Others view the lower sensitivity as an attribute, because some people who receive positive PCR test results are infected, but are no longer able to spread the virus to others. So antigen tests could shift the focus to identifying the most infectious people.
At present, antigen tests are administered by trained professionals, but some companies are developing versions that are simple enough to be used at home—similar to pregnancy tests.
“Making the tests faster, cheaper, easier is definitely the goal—and I think the antigen test is the way to get there,” says Martin Burke, a chemist at the University of Illinois at Urbana-Champaign, who is co-developing rapid tests, including antigen-based assays. “This is by no means the perfect solution, it’s just the fastest thing we could get going now,” he says.
What tests are there and how do they work?
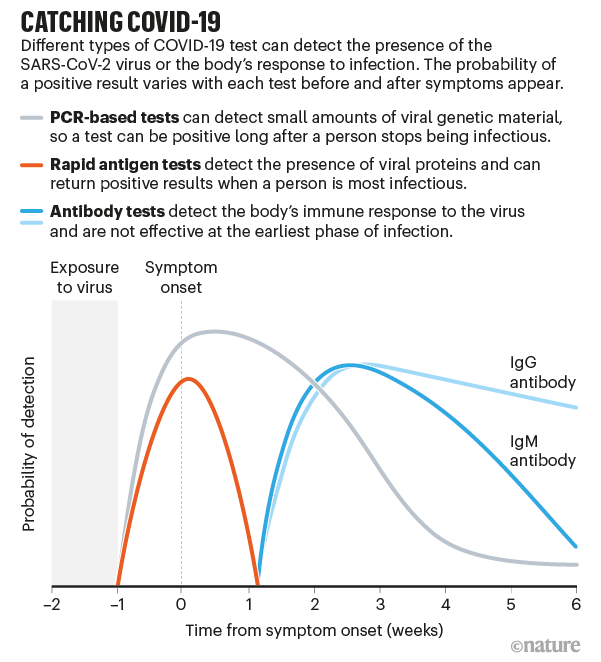
Tests for COVID-19 fall into two categories: diagnostic tests such as PCR and antigen assays, which detect parts of the SARS-CoV-2 virus, and antibody tests that sense molecules that people produce when they have been infected by the virus. Antibodies can take several days to develop after an infection and often stay in the blood for weeks after recovery, so antibody tests have limited use in diagnosis (see ‘Catching COVID-19’).
The high-sensitivity PCR tests are almost 100% accurate in spotting infected people, when they are administered properly. But such tests generally require trained personnel, specific reagents and expensive machines that take hours to provide results.
Countries such as South Korea and New Zealand have succeeded in boosting PCR-based testing, but scaling up these tests has proved difficult elsewhere. The United States, for example, has seen a slow and poorly coordinated response to outbreaks, faulty tests from the Centers for Disease Control and Prevention (CDC) and problems with the supply chain. All of this has hindered efforts to collect and process samples for PCR, pushing waiting times to days or even weeks. These delays, along with a lack of tests, have contributed to the rampant spread of COVID-19 across the country, which by 16 September had seen almost 200,000 deaths from the disease.
A typical antigen test starts with a health-care professional swabbing the back of a person’s nose or throat—although companies are developing kits that use saliva samples, which are easier and safer to collect than a swab. The sample is then mixed with a solution that breaks the virus open and frees specific viral proteins. The mix is added to a paper strip that contains an antibody tailored to bind to these proteins, if they’re present in the solution. A positive test result can be detected either as a fluorescent glow or as a dark band on the paper strip.
Antigen tests give results in less than 30 minutes, don’t have to be processed in a lab and are cheap to produce. Yet that speed comes with a cost in sensitivity. Whereas a typical PCR test can detect a single molecule of RNA in a microlitre of solution, antigen tests need a sample to contain thousands—probably tens of thousands—of virus particles per microlitre to produce a positive result. So, if a person has low amounts of virus in their body, the test might give a false-negative result.
When used on people who were positive for SARS-CoV-2 in a standard PCR test, Abbott’s antigen assay correctly spotted the virus in 95–100% of cases if the samples were collected within a week of the onset of symptoms. But that proportion dropped to 75% if samples were taken more than a week after people first showed symptoms. The sensitivity—or the rate of detecting infections correctly—of the other antigen tests used in the United States is between 84% and 98% if a person is tested in the week after showing symptoms.
Companies and academic research labs are also rolling out other tests that are faster, cheaper and more user-friendly than standard PCR assays, although they are not being produced on the same scale as antigen tests. Some of these other tests use the gene-editing tool CRISPR to zero in on genetic snippets of the coronavirus. Others are quicker variants of the PCR test that use different reagents, meaning they’re not limited by the same supply-chain problems. Saliva-based PCR tests, for example, are being used as screening tools in universities and for professional basketball teams.
Which tests tell whether someone is infectious?
Although the PCR method can test whether someone is infectious, it also detects people who have the virus but are not likely to spread it.
Antigen-based testing, by contrast, could help to rapidly identify people who have high levels of virus—those who are most likely to be infectious to others—and isolate them from the community, says Marion Koopmans, a virologist at the Erasmus University Medical Centre in Rotterdam, the Netherlands. “The question is, what is the safe limit? Because the moment you get that wrong, the whole idea implodes,” she says. It’s still unclear what viral load is the threshold below which a person is no longer contagious, says Koopmans, who is working with the World Health Organization (WHO) to determine a standard to validate rapid tests. “It would be very worrying if everyone does that on their own, using different criteria,” she says.
Viral load peaks early in SARS-CoV-2 infections and then gradually declines, with tiny amounts of virus RNA staying in someone’s nose or throat for weeks or possibly months. And although there are not enough data to equate different viral levels with how infectious people are, there is evidence that individuals are unlikely to spread the virus about eight to ten days after showing symptoms.
“If you’re at risk of transmitting the virus to somebody else, you’re going to have plenty of viral particles—those would certainly show up in antigen tests,” says Michael Mina, an infectious-disease immunologist at the Harvard T. H. Chan School of Public Health in Boston, Massachusetts, who has been a vocal proponent of antigen tests.
There are challenges at the start of the infection, when people have low levels of the virus. The answer, says Mina, is frequent testing—done multiple times per week. This could quickly identify infected people, even if the assays are less sensitive than a PCR-based test, because the amount of virus in their noses and throats rises within hours, he says.
Mina and his colleagues have used statistical models to assess this strategy. In a preprint updated on 8 September, they suggest that testing people twice a week with a relatively insensitive test could be more effective at curbing the spread of SARS-CoV-2 than are more-accurate tests done once every two weeks. Another study that modelled different scenarios for safely reopening university campuses reported similar findings.
To slow outbreaks, the focus should be on identifying those who are at risk of spreading SARS-CoV-2 to other people, rather than on spotting anyone who is infected with it, some experts say.
When used as a screening tool to frequently assess as many people as possible, rapid antigen tests could be “a game changer”, says Rebecca Lee Smith, an epidemiologist at the University of Illinois.
How do countries plan to use antigen tests?
At the beginning of April, as coronavirus outbreaks raged across the world, India had tested only about 150,000 people—one of the lowest testing rates per capita worldwide. On 21 August, the country conducted more than one million coronavirus tests in a single day. It reached that milestone after Indian authorities began using antigen assays to boost testing capacity.
Delhi was the first Indian state to begin using rapid antigen tests, in June. By mid-July, the number of cases there had decreased and the daily death counts had plateaued, suggesting that the tests might have played some part in controlling the spread of the virus. Epidemiologist K. Srinath Reddy, president of the Public Health Foundation of India, a non-profit organization in New Delhi, says that the Delhi example is interesting, but not clear-cut: he notes that the government started to lift lockdown restrictions in August, which led to a surge in infections. “Rapid antigen tests have picked up the increased number of cases, but whether they have been successful in limiting the spread of COVID, we’ll only know in the next couple of months,” Reddy says.
So far, India has approved the use of three antigen tests for screening large numbers of people, whether or not they have symptoms. One of the kits was evaluated by the Indian Council of Medical Research (ICMR) and the All India Institute of Medical Sciences, which found that the test detected infections between 51% and 84% of the time. Guidance from the ICMR says that people who have a negative result from an antigen test should also get a PCR test if they show symptoms, to rule out the possibility that the rapid test missed an infection.
The WHO and the US CDC have also advised getting a PCR test if people showing symptoms test negative with a rapid antigen test. The US FDA has so far granted emergency use authorization for four antigen tests, each of which has a higher sensitivity than those used in India. The 150 million tests bought from Abbott will be used in schools and “other special needs populations”, according to the Department of Health and Human Services. The FDA, however, has authorized antigen-based tests only for people who have had symptoms for 12 days or fewer. Tests must be prescribed by a physician and administered by a health-care professional.

Other countries are also considering the use of rapid antigen tests to meet targets. In July, the Philippine Society for Microbiology and Infectious Diseases issued temporary guidelines for clinicians and health-care workers, saying that antigen tests could be used as an alternative to PCR for diagnosing a coronavirus infection during the first week in people with symptoms. But it also recommends that all negative results should be confirmed with a PCR-based assay, says Edsel Salvaña, an infectious-diseases expert at the University of the Philippines Manila, who is advising Philippine officials on rapid testing.
Antigen-based tests are being used in some of Italy’s major airports to screen people who arrive from four Mediterranean countries considered to have a high risk of infection. Negative results do not have to be confirmed with a PCR test. The Italian health minister, Roberto Speranza, has announced plans to use antigen tests to screen passengers at all of the country’s airports, and a group of experts has urged the Italian government to use the rapid tests in schools and universities.
But others don’t think rapid antigen tests are a good idea. When trying to contain small outbreaks, such as those happening in Italy, public-health authorities should use assays that are highly accurate, because missing even just one positive individual could lead to a steep increase in the total number of cases, says Andrea Crisanti, a microbiologist at the University of Padua.
Some researchers worry that there won’t be enough antigen tests available to greatly expand their use. “Rapid tests right now are for the happy few,” Koopmans says. “If we want to take these assays responsibly forward, we should talk about whether they can be produced to levels that would make them globally available.”
Could antigen assays be used at home like pregnancy tests?
Several experts have promoted the idea of developing an antigen test that is cheap and simple enough to use at home, without a health-care worker administering it.
Burke says what’s needed is something as easy as a pregnancy test. “You just spit into a tube, put a piece of paper in it and you get the result within minutes,” Burke says. “Testing should become a part of life: in the morning you take your cereals, your vitamins, and you quickly check your status,” he says.
A few companies are developing simple paper-strip antigen tests. But drug regulators have not yet approved them for emergency use. “We don’t have a lot of real-life experience with these tests, and a lot of the validations have only been done in the laboratory,” Salvaña says.

Beyond concerns about costs and availability, researchers worry that, with an over-the-counter test, people who get positive results might not follow up with public-health authorities, so their contacts won’t be traced. Another risk would be people “gaming the system”, Smith says—for example, getting someone else to take their test—so they can be sure of a negative result and avoid quarantine. Without incentives such as freely available tests and a living salary for those who have to isolate, testing and self-isolation could become a luxury reserved for wealthier people, others have argued.
Another concern is that people will get a false sense of security from tests that have only limited accuracy. “There’s a big risk that the moment these tests become widely available, people will just use them and say, ‘It’s negative, so I’m clear,’” Koopmans says.
Even when testing negative, people should continue to wash their hands, wear masks and avoid gathering in big groups, she says. Testing, she adds, “cannot replace the basic control measures that need to be in place to keep this virus controlled”.
This article is reproduced with permission and was first published on September 16 2020.