From the embryonic stages to late life, cells often make incredible journeys, sometimes even traversing an entire organism. They reach their destination by chemotaxis, following signals that lead them to the goal like a chemical yellow brick road. The catch is that different levels, or gradients, of a chemical drawing cells to a target only work over short distances. What guides them over the hills and valleys of a longer journey through the body has been unclear.
Findings reported on August 27 in Science show how two kinds of cells—one a dirt-dwelling amoeba and the other a mouse cancer cell line—manage these seemingly impossible journeys. The key is that the cells do not work along a preexisting gradient but create one themselves by breaking down the chemical lure as they encounter it. Like gathering string while finding one’s way out of a labyrinth, the chemical-free path they leave behind keeps them binding to—and following—the guide in front of them.
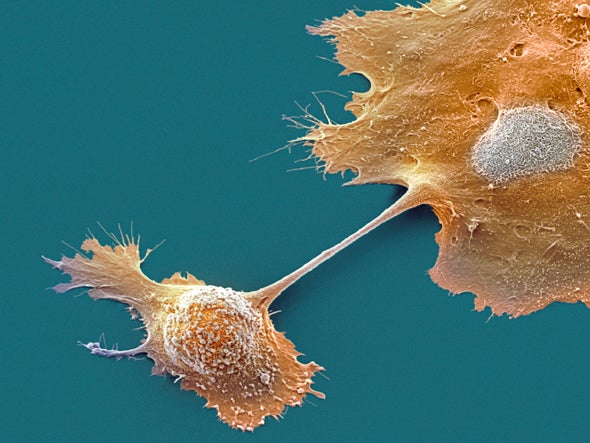
Take melanoma cells, which are among the most avidly metastasizing tumor cells. Once they have broken down a chemoattractant called lysophosphatidic acid locally in the tumor, they move toward higher levels of the molecule away from the tumor. The cells forge a path into the bloodstream, where lysophosphatidic acid is relatively uniformly distributed. The melanoma cells break the chemical down as they go, leaving a high concentration of it in front of them that they follow and consume, Pac-Man-like, whereas low levels remain behind.
The research team confirmed the use of this tactic in both an amoeba and a mammalian cell line, suggesting a commonality among cells engaged in long-distance orienteering. That outcome “is really interesting and demonstrates that self-generated gradients are a universal mechanism for steering directional migration of groups of cells for long distances,” says Pablo Sáez, a professor and group leader in the department of biochemistry and molecular cell biology at the University Medical Center Hamburg–Eppendorf in Germany, who was not involved in the work. He adds that the result highlights the usefulness of some of the techniques the researchers used, including mathematical modeling to predict how the cells might behave and employing mazes to test those predictions.
In fact, Luke Tweedy, a postdoctoral research associate at the Beatson Institute for Cancer Research in Scotland and his colleagues reasoned that following a winding path through the complex topography of an organism might be a lot like navigating a labyrinth. To test their idea, they used two kinds of cells: the amoeba Dictyostelium discoideum, or “Dicty” for short, and mouse pancreatic cancer cells. Dicty cells were especially of interest because of their proficiency at breaking down the chemical yellow brick road as they travel it so that the right path is always before them. With this tendency to “gather string” as it moves along, Dicty was an exemplary candidate for maze solving—a “chemotactic prodigy,” as Tweedy puts it.
Tweedy and his colleagues found that Dicty lived up to its reputation, rapidly solving a complex maze in an hour that could take the tortoiselike pancreatic cancer cells several days. The researchers tested the cells in a lot of different mazes, some with shorter versus longer dead ends and different forks. When cells faced a choice between a dead end and a true path, a few wayward ones would dispatch all of the chemoattractant trapped in the cul-de-sac, and the rest of them would orient to the other fork that was still flowing with the alluring molecules.
The most memorable test the investigators used was the one they modeled on the famous maze at Hampton Court Palace near London. They chose it, Tweedy says, for the “razzle-dazzle” and to “capture the imagination.” Dicty, the prodigy protist, not only solved this maze but also managed to use its self-generating gradient skills to find a shortcut.
Researchers also brought in computational models to predict cell behavior, which could have implications for human conditions that involve migrating cells. An example is human cancer cells that have something in common with amoebas—whether that connection involves the normal migration of immune cells or the pathological journey of metastatic cancer cells “They use the same fundamental mechanism of migration, [in which] receptors detect attractants and guide the cytoskeleton to move the cell.”
In fact, the similarities are strong enough that Tweedy sees many ways to bridge the amoeba-human-cell gap, including applying maze-solving ideas to predicting the path that the cancer cells of glioblastoma follow.
The results could also offer a rare window into some early processes in mammalian embryos. The cells that eventually set up shop in the gonads start far away from their target early in an embryo’s development. These so-called germ cells have to move over embryonic hill and dale to get to the appropriate destination. If the behavior of Dicty or the much slower pancreatic cancer cells is universal, then these germ cells may use a similar tactic to get to the future gonads and avoid taking a wrong turn toward, say, the gut. The implication is that building complex organisms sometimes means that cells only get where they are going by making their own way.
A version of this article with the title “Cells in a Maze” was adapted for inclusion in the November 2020 issue of Scientific American.