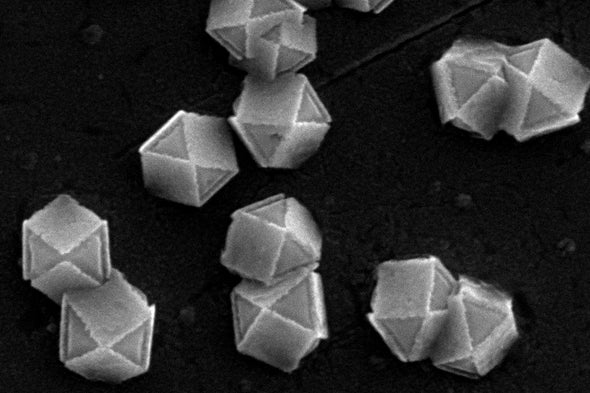
For the first time, researchers have used light to control the shape of nanoparticles and create micron-size hollow shells from crystals of cuprous oxide (copper and oxygen). Such particles could have future applications as a low-cost catalyst to help pull excess carbon dioxide from the air, a way to improve microscopic imaging and more, says Bryce Sadtler, a chemist at Washington University in St. Louis and senior author of a study on the new method, published last October in Chemistry of Materials.
The hollowing process involves visible light, an alkaline solution and a source of voltage, Sadtler explains. Illuminating a cuprous oxide microcrystal excites its electrons, which join with copper ions to form regular copper atoms. No longer bound to oxygen, these atoms are free to jump to the particle’s surface and form a copper metal coating that shields parts of the underlying crystal from the solution.
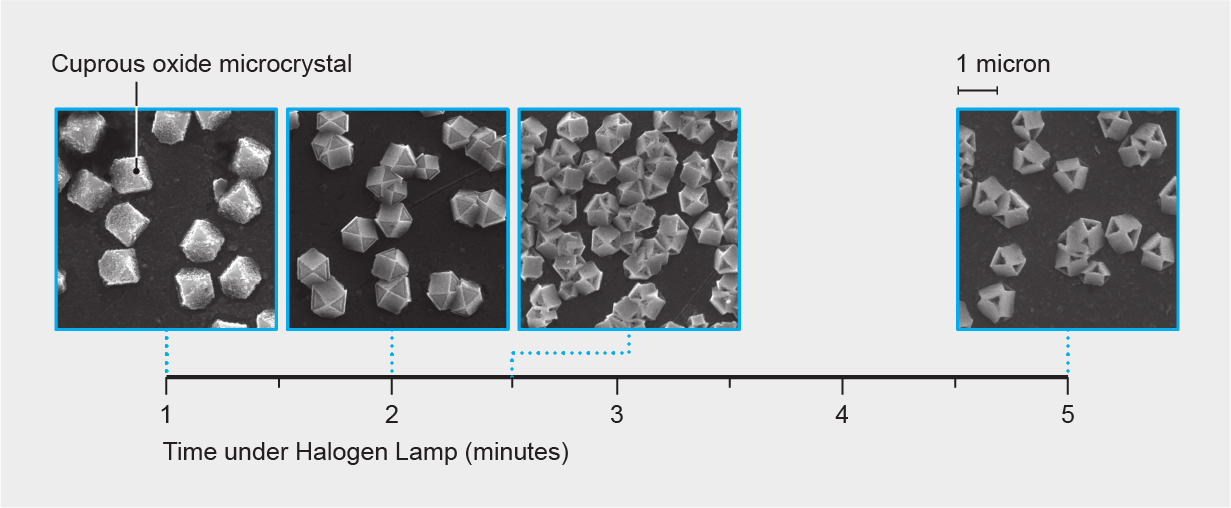
The crystal’s structure determines which of its faces are protected and which dissolve: Some faces’ atomic makeup lets electrons get excited more easily, bringing metal atoms to the surface. But the unprotected faces dissolve quickly, shaping the crystal along stark, geometric lines. “A diamond can only be [easily] cut a certain number of ways” for similar reasons, Sadtler says. Diamonds break most easily in line with rows of atoms in their crystal structure.
Stephen Maldonado, a chemist at the University of Michigan, who was not involved in the study, says the group’s findings “could be potentially useful in terms of designing catalysts for high-efficiency ... CO2 reduction, or something else.”
The large surface area and specific shape of the hollowed-out crystals could also be useful beyond facilitating a carbon-capture reaction, Sadtler says. In microscopic imaging, for example, existing methods are great for identifying solid, crystalline materials—but they struggle to identify biological molecules. According to Sadtler, similar hollowed structures could surround organic molecules, possibly in blood or urine samples, and boost the signal of the hard-to-detect matter. The researchers are also investigating different materials that strongly interact with light, such as iron and manganese oxides, which hold promise for hydrogen fuel-cell technology.