One of the two legal definitions of death is irreversible cessation of all brain function, commonly known as “brain death.” (The other is the halting of circulatory and respiratory function.) It was widely believed that brain cells undergo rapid—and irreversible—degeneration immediately after death. But a striking new study, published Wednesday in Nature, suggests that much functionality can be preserved or restored—even hours after death. A research team, based primarily at the Yale School of Medicine, managed to revive some functions in the whole brains of pigs slaughtered four hours previously and to sustain them for a further six hours.
The work was motivated by the observation that cells can be harvested from postmortem brains and sustained in cell cultures for study, neuroscientist and team leader Nenad Sestan said in a press briefing: “In short, if we can do this in a petri dish, can we do it in an intact brain?” The system Sestan and his colleague developed, called BrainEx, comprises three elements: a computerized system of pumps, filters and reservoirs; a blood substitute containing no cells but capable of carrying oxygen, along with numerous compounds designed to protect cells; and a surgical procedure to hook everything up.
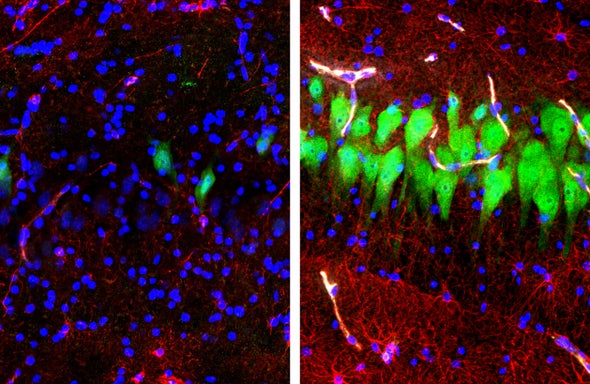
The researchers compared brains they sustained using BrainEx with brains that were perfused with an inert fluid or that were not hooked up to anything to assess their relative states at different times. The system reduced cell death, preserved anatomical integrity, and restored circulatory, metabolic and some cellular functions. The team was even able to observe inflammatory responses from immune cells, called glia, by introducing a molecule that mimics bacterial infection. The findings suggest that cells are far more resilient to the damage caused by stopping blood flow, which deprives the brain of oxygen (known as ischemia), than previously appreciated. “We didn’t have any a priori hypothesis that we’d be able to restore cells to this level,” Sestan told journalists. “We were really surprised.”
This work could represent a major contribution to methods available for studying the brain. The research was funded under the National Institutes of Health’s BRAIN (Brain Research through Advancing Innovative Neurotechnologies) Initiative, and NIH experts also briefed the press. “This is a real breakthrough for brain research; it’s a new tool that bridges the gap between basic neuroscience and clinical research,” said Andrea Beckel-Mitchener, a BRAIN Initiative team leader at the National Institute of Mental Health. “It provides experimental access like we’ve never had before; we anticipate interesting studies on brain circulation, cell metabolism, other cell biology and mapping long-range connections.”
The immediate findings have implications for how we understand brain death. “It’s drilled into us as scientists and doctors that after even just a couple of minutes, there’s no going back; this clearly turns that on its head,” says Madeline Lancaster, an expert in research on brain organoids (so-called “mini brains” grown from stem cells—at the University of Cambridge, who was not involved in the work. “Where I see the most potential in the short term is just changing thinking about that and hopefully spurring people to do more research into humans who are potentially brain dead—and understanding how we might be able to bring them back.” Extending the time before declaration of brain death has other implications—it could delay when organs could become available for donations, as discussed in a commentary article in Nature. One near-term benefit is the opportunity to learn more about ischemic injury. “We hope to better understand how brain cells react to circulatory arrest and if we can intervene and salvage these cells,” Sestan said. “By doing this, we can possibly come up with better therapies for stroke and other disorders that cause brain cells to die.”
In the longer term, the system could provide a powerful method for studying brain connectivity, circuit function and disease processes. A certain amount can already be learned using brain slices, brain organoids (so-called “mini-brains” grown from stem cells) and postmortem brains, but this system offers at least two advantages: First, an intact brain offers an unparalleled opportunity to study brain circuitry. “If the question is one where you really need the context of the whole organ, this definitely gives you an advantage,” Lancaster says. “If we knew [brain circuits] were functional to some degree, being able to look at a fully intact circuit would be very powerful.” Second, postmortem studies limit observations to discrete points in time, which curbs understanding of how diseases progress. For instance, neurodegenerative diseases such as Alzheimer’s are thought, by some, to involve toxic proteins spreading in the brain. “You could do a lot more here in terms of perturbing the brain in various ways: introducing a prion protein, or amyloid-beta, for example, and looking at spreading,” Lancaster says. “Being able to see it in real time is really key. This would be a way to do that.”
The team engaged with existing ethics frameworks from the time it began to plan the experiments. Chief among the ethics concerns is whether resuscitated brains might exhibit signs of consciousness. The study specifically wanted to avoid the remote possibility that consciousness would return, and the researchers were prepared to lower temperatures and deploy anesthetics to extinguish such signs if they emerged. They continually monitored electrical recordings from the brains’ surface and saw no evidence of the global electrical activity that would be expected if there was anything approaching cognition. “I’m very confident no consciousness was present in these restored brains,” says Christof Koch of the Allen Institute for Brain Science in Seattle, a leading researcher in the neuroscience of consciousness. There were none of the signals we associate with consciousness, or even sleep, Koch says: “Only a flat line, implying a complete absence of any sort of consciousness.”
But part of the reason for the lack of electrical activity may relate to the fact that the perfusion solution contained neural activity blockers. The researchers included these blockers because they wanted to keep the brains quiescent to maximize cellular recovery. An active brain would require a massively greater energy supply, and the very act of firing can damage neurons (a phenomenon known as excitotoxicity). The team took tissue samples to show that individual neurons were still electrically functional, which necessarily involved washing out the solution to prepare the samples for electrophysiological recordings.
But what would have happened if these blockers were not used? “We cannot speak with any scientific certainty to that point, since we didn’t run those experiments,” Stefano Daniele, co-lead author of the study, told journalists. If such future experiments were to move resuscitated brains closer to conscious activity, that would spur discussions about what can be considered truly dead. These considerations are discussed in another accompanying commentary article co-authored by legal scholar Nita Farahany, a bioethicist who is a member of the Neuroethics Working Group at the BRAIN Initiative and who the researchers consulted from an early stage.
The team also consulted the Institutional Animal Care and Use Committee (IACUC) at Yale, and the members were told the study was not subject to animal welfare-protection guidelines. Most obviously, the pigs were already dead: the researchers procured the brains from a pork processing plant, so no animal was sacrificed for this research. In any case, such guidelines do not apply to animals raised for food.
Moving forward, the work must be replicated by other labs that will have to learn the intricacies of the manually operated system. The team itself wants to establish how long brains can be sustained this way. The perfusion stage of the experiment only lasted six hours because at that point, the control brains that were not in the BrainEx system had undergone too much disintegration for meaningful comparisons to be made.
If brains can be kept going for long periods, and researchers turn from prioritizing cellular recovery to reviving electrical function in situ, that would enter uncharted ethical territory. “There are some questions that need to be answered first,” Farahany says. “Can we ever get EEG [electroencephalogram] recovery? What are the limits to it if we ever get to that? And what are the implications, then, for animal research and for human research one day?” In Farahany’s view, these unknowns put what was initially considered dead tissue into a new ethical category. “It’s the potential [for greater recovery] that creates a moral status that’s different and requires that we treat it differently,” Farahany says. “One could go with the safest possible approach there, which is to give it some or similar protections that would be accorded to animal research subjects.” Such an experiment would likely be first tried in rodents, initially by just removing the chemicals that block electrical activity. If anything that looked remotely like conscious activity were to be detected, we would be in territory for which new ethical guidelines would be needed. “At that point, if you start thinking about it more like a living animal, then minimizing any risk of pain or distress would be appropriate,” Farahany says. “The problem is: right now, we think of this as tissue research and it's no longer just clearly dead. It’s just not exactly alive either.”